1. Background
In December 2019, pneumonia related to the 2019 novel severe acute respiratory syndrome (SARS)-CoV-2 (2019-nCoV) was reported in Wuhan, China (1). Human SARS-CoV is one of the major pathogens of respiratory infection (2). The two most pathogenic viruses, SARS-CoV and Middle East respiratory syndrome (MERS)-CoV, induce severe respiratory syndrome in humans, and four human coronaviruses, including HCoV-OC43, HCoV-229E, HCoV-NL63, and HCoV-HKU1, cause mild upper respiratory disorders (1). The SARS-CoV depends on the host cell for replication and enters the host cell with protease activity (3). Therefore, natural compounds with antiprotease activity can neutralize the effect of SARS-CoV (4). The Otostegia genus possesses 31 species and is a member of the Lamiaceae family, which grows in the Asian and Mediterranean regions. Lamiaceae species are used traditionally (5) for the treatment of many chronic diseases (6); they possess a diterpenoid component (otostegindiol), and many usages of this compound are observed (7). Moreover, the species of the genus Otostegia are widely used by traditional Persian medicine practitioners for the treatment of various disorders, and its components have been shown to possess antispasmodic, antidepressant, antiulcer, anxiolytic, and sedative properties (7). One of the Otostegia species is Otostegia persica, which is traditionally consumed in some parts of Iran as an antidiabetic plant. Otostegia persica is used to treat diabetes in both modern and traditional medicine (8). Thus, its reported potential has been examined by some researchers. Also, diterpenoids such as otostegindiol are compounds that have been separated from the aerial parts of O. persica. Ayatollahi et al. (9, 10) demonstrated that a dichloromethane extract of aerial parts of O. persica could be a significant source of various terpenoids.
Triterpenes are commonly biosynthesized following the isoprene rule of Ruzicka. Triterpenes have been reported to have a broad range of therapeutic uses, including antiviral activities (11). Moreover, some of the significant constituents of O. persica are flavonoids and phenolic compounds, including morin, quercetin, kaempferol, and isovitexin (C-glucoflavone), as well as trans-cinnamic acid (12). Another species of Otostegia is O. aucheri, which exhibited anti-hyperglycemic properties (8). It is reported that O. aucheri possesses 6,8-di-C-glycosylapigenin and friedelin (8). Recent studies have also exhibited the antiviral activity of apigenin against several viruses (13). In addition, Khandelwal et al. have reported that apigenin possesses antiviral effects against the buffalopox virus (14). Another investigation reported that apigenin analogs bind to COVID-19's main protease substrates. The interactions between the analogs of apigenin and SARS-CoV-2 Mpro inhibitors were detected as electronic bonds, hydrogen bonds, and hydrophobic interactions. Several studies show that apigenin 7-glucoside-4’-p-coumarate is the best candidate for SARS-CoV-2 Mpro inhibition. Apigenin (4′,5,7-trihydroxyflavone), a flavone glycoside, is found in a variety of vegetables and fruits (15). Antiprotease activities of some plants have been reported (16).
2. Objectives
This study evaluated the kinetic parameters (Km, Vmax) of shrimp protease as a 3CLpro model inhibition and determined the mode of inhibition of O. persica and O. aucheri extracts and different fractions.
3. Methods
3.1. Materials
One material, Potassium sodium tartrate (Cat. No. 217255), was obtained from Sigma Aldrich, USA. Trichloroacetic acid (10% w/v, Cat. No. 76-03-9) was purchased from Merck Chemicals (Darmstadt, Germany). All chemicals used, such as casein (1% w/v, Cat. No. C7078), tris (Cat. No. 77-86-1), and di-potassium hydrogen phosphate-3 hydrate (Cat. No. 16788-57-1), were of analytical grade (≥ 99% purity). Casein was dissolved in a pH 7.4 phosphate buffer.
3.2. Specimen Collection
Otostegia persica and O. aucheri were gathered from around Bandar Abbas, Hormozgan province, Iran, by Mr. Asadipour in March 2020. These plants were identified by the botanists at Shiraz University of Medical Sciences and were then extracted. The voucher number was saved in the Medicinal Plants Processing Research Center, School of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran (MPRCM 94-86, and MPPRC-96-9 for O. persica and O. aucheri, respectively).
3.3. Extraction and Fractionation of the Studied Plants
First, the leaves of the two plants (3000 g per species) were air-dried at 25°C, chopped, and macerated in 80% v/v ethanol for 72 hours at room temperature. Then, the extracts were concentrated using a rotary evaporator (Eyela model N-1100, 40°C, 100 mbar). Subsequently, dried extracts were suspended in a 70:30 methanol:water solution and then filtered through Whatman No. 1 filter paper. After extraction, these extracts were subjected to fractionation by various solvents in order of increasing polarity; in other words, fractionation was performed with petroleum ether (petr), chloroform (chlo), ethyl acetate (eta), and n-butanol (buta), respectively (9). Ethanol extracts, petr fractions, chlo fractions, eta fractions, and buta fractions yielded 166.5, 80.7, 19.9, 15.1, and 1.1 g from 3000 g of powdered plant, respectively. The source of the enzyme was shrimp-Penaeus vannamei protease as a model for 3CLpro. Professor Ahmad Homaie (Hormozgan University, Bandar Abbas, Iran) donated the protease.
3.4. Determination of Protease Inhibition
Two hundred microliters of phosphate buffer (50 mM, pH = 7.4) was blended with 200 μL of cysteine-like protease from shrimp. Then, 400 μL of casein (1%, Cat. No. 9000-71-9), as a substrate, was added, and the mixture was incubated for 10 minutes at 25°C. Afterward, 800 μL of trichloroacetic acid (TCA, 10%) was added as a stopper of the reaction. After 21 minutes, the mixture was centrifuged (Peco FD-T6-4000, Iran) at 12000 rpm for 5 minutes to ensure complete precipitation, and a UV-visible spectrophotometer (PG instrument T90, England) was used to determine the absorbance of the supernatant (17) at 280 nm ± 2 nm.
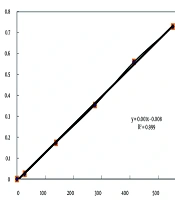
Protease inhibitory activity was determined as follows: Inhibition (%) = 100 - [(OD sample - OD blank) / (OD control - OD blank) × 100]. The above formula was adapted from Kunitz, 1947 (18). A blank solution was prepared to omit the background absorbance and contained plant extract without the enzyme. In the control solution, the plant extract was replaced with ethanol. The control contained the enzyme without the plant extract. The absorbances of 0.1 to 550 micromol/mL of tyrosine were detected at 280 nm by spectrophotometer, and the standard curve was plotted (Figure 1).
3.5. Kinetics of Protease Inhibition
The inhibition modes of the protease were evaluated based on the method delineated by Rouzbehan et al. (9). For this evaluation, constant amounts of protease were blended with increasing concentrations of its substrates (casein concentrations from 0.1 to 2.0 mg/mL) at 25°C for 10 minutes in a pH 7.4 phosphate buffer, and afterward mixed with and without the IC50 concentrations of the samples. Then, the reactions were stopped, and absorption was detected using the Michaelis-Menten curve. After that, the absorbance was converted into the activity of the enzyme using the standard curve of tyrosine (Figure 1); then, different concentrations of the sample were plotted against the concerned activity. All assessments were repeated three times. The Michaelis-Menten equation is: V = Vmax [S] / (Km + [S]).
3.6. Statistical Analysis
The results are shown as the mean ± SD of the biological triplicates. One-way Analysis of Variance (ANOVA) with repeated measures and post-hoc Tukey (α = 0.05) were employed to detect the variations among the means. A P-value of less than 0.05 was regarded as statistically significant. After that, kinetic parameters including Vmax and Km were determined using GraphPad Prism 7.0. For this determination, we principally converted the amount of reading absorption into enzyme activity using the product (tyrosine) standard curve. Next, the values of Km and Vmax were determined employing GraphPad Prism 7.0. Statistical analysis was done with GraphPad InStat 3.0 software.
4. Results
4.1. The Yield of the Extraction
In both Otostegia species, 3000 g of dried leaves yielded 166 g of dried extract. The yields were 5.55% for ethanol extracts of O. aucheri and O. persica. Additionally, 3000 g of petr, chlo, eta, and n-buta fractions gave 80.7, 19.9, 15.14, and 1.1 g of lyophilized post-fractionation, respectively. The yields ranged from 1.1 g (n-buta) to 80.7 g (petr) across replicates. Yield percentages of petr, chlo, eta, and buta fractions were 2.69, 0.66, 0.50, and 0.06, respectively.
4.2. The Effects of the Extracts and Fractions of Otostegia persica and Otostegia aucheri in Inhibition of Protease
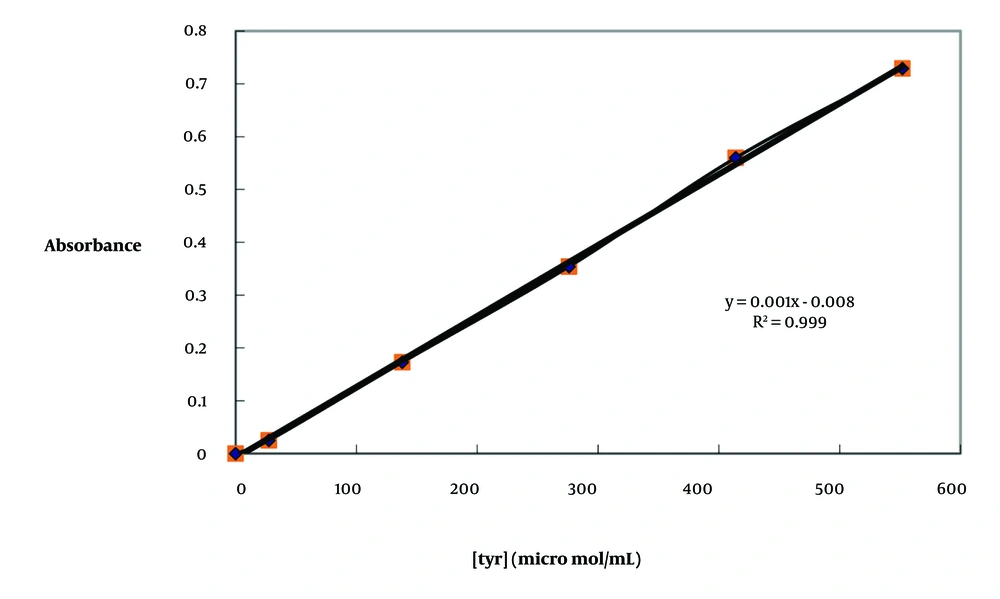
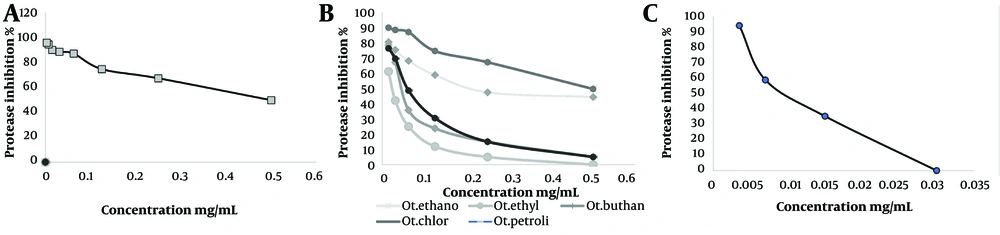
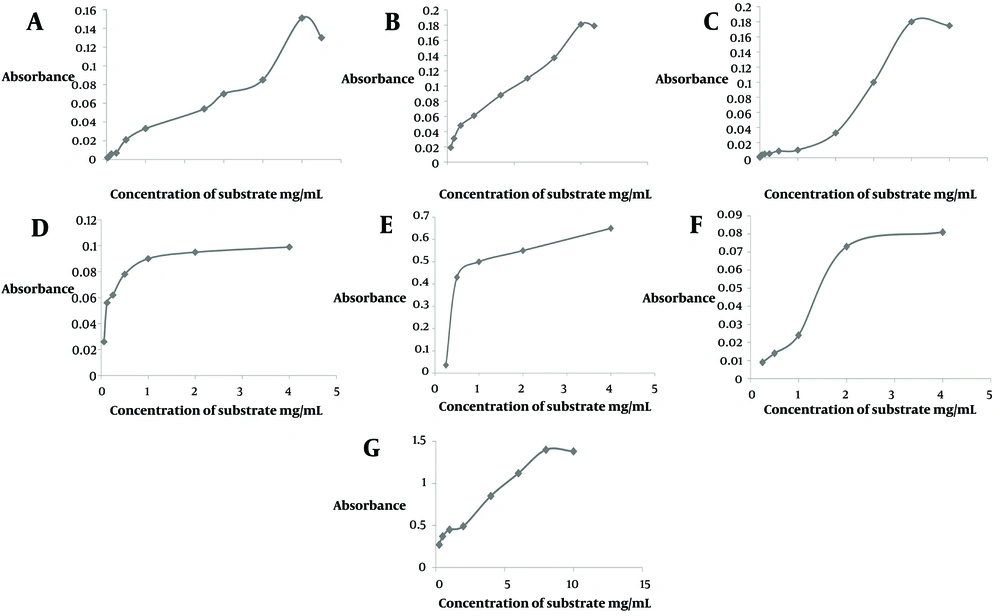
Samples tested ranged from 0.015 to 0.5 mg/mL. The results of the present research showed that at 0.125 mg/mL, a buta fraction of O. persica had 31.2 ± 4.03% inhibition on protease activity, and at 0.25 mg/mL, a petr fraction of O. persica had 37.05 ± 4.8% inhibition on protease activity. Other fractions of O. persica had no effects on the inhibition of protease (Figure 2A). Additionally, the IC50 of O. persica extract was 0.46 ± 0.005 mg/mL (Table 1). However, the eta fraction of O. aucheri had the most inhibitory effect on protease activity in comparison to iodoacetamide (IC50 0.002 ± 0.0001 mg/mL, P = 0.04, Table 1). Besides, buta and chlo fractions of O. aucheri also had significant inhibitory effects on protease inhibition (Figure 2B). Figure 2C showed the percentage of protease inhibition of iodoacetamide as a standard. The saturation diagrams (Michaelis-Menten curve) of protease inhibition are shown in Figure 3A - G. All experiments were performed three times.
A, percentage of protease inhibition of Otostegia persica extract (sample sizes, n = 3 per concentration); B, percentage of protease inhibition of O. aucheri extract and its fractions. Ot. Ethanol, ethanol extract of O. aucheri; Ot. Petroli, petroleum ether (petr) fraction of O. aucheri; Ot. Chlor, chloroform (chlo) fraction of O. aucheri; Ot. Ethyl, ethyl acetate (eta) fraction of O. aucheri; Ot. Buthan, butanol (buta) fraction of O. aucheri (sample sizes, n = 3 per concentration); C, percentage of protease inhibition of iodoacetamide as a standard (sample sizes, n = 3 per concentration).
| Samples | Maximum of Protease Inhibition % at 0.015 mg/mL of the Sample | IC50 mg/mL | Vmax ± SD (µmol/min) b | Km ± SD (mmol) c | Kind of Inhibition |
|---|---|---|---|---|---|
| Otostegia persica extract | 88.95 ± 1.3 | 0.46 ± 0.005 | 1.03 ± 0.07 | 0.0125 ± 0.003 | Uncompetitive |
| O. aucheri extract | 88.6 ± 1.9 | ND | ND | ND | ND |
| Petr fraction of O. aucheri | 79.3 ± 1.6 | 0.325 ± 0.007 | 1.2 ± 0.005 | 0.03 ± 0.003 | Mixed d |
| Chlo fraction of O. aucheri | 77.15 ± 1.4 | 0.09 ± 0.007 | 1.12 ± 0.007 | 0.019 ± 0.0007 | Uncompetitive |
| Eta fraction of O. aucheri | 61.5 ± 0.6 | 0.002 ± 0.0001 | 1.19 ± 0.2 | 0.025 ± 0.004 | Uncompetitive |
| Buta fraction of O. aucheri | 77.95 ± 0.5 | 0.07 ± 0.002 | 1.15 ± 0.15 | 0.025 ± 0.003 | Uncompetitive |
| Iodoacetamide (standard) | At 0.03 mg/mL of iodoacetamide possessed 98.2 ± 2.2 % inhibition | 0.01 ± 0.002 | 1.13 ± 0.002 | 0.024 ± 0.003 | Uncompetitive |
| Without inhibition | 1.2 ± 0.003 | 0.031 ± 0.002 |
Abbreviations: ND, not determined; petr, petroleum ether; chlo, chloroform; eta, ethyl acetate; buta, butanol.
a Values are expressed as mean ± SD.
b Vmax values of Otostegia persica extract and petr, chlo, and eta fractions of O. aucheri were significantly different in comparison control (reaction without inhibition; P < 0.05). Vmax values of buta fraction and iodoacetamide were not significantly different in comparison control (P > 0.05).
c Km values of all of the samples and iodoacetamide were not significantly different in comparison control (P > 0.05).
d Mixed inhibition such as petr fraction, altered both Km and Vmax.
A, Michaelis-Menten curve of ethanol extract of Otostegia persica; B, Michaelis-Menten curve of the control; C, Michaelis-Menten curve of the petroleum ether (petr) fraction of O. aucheri; D, Michaelis-Menten curve of the butanol (buta) fraction of O. aucheri; E, Michaelis-Menten curve of the chloroform (chlo) fraction of O. aucheri; F, Michaelis-Menten curve of the ethyl acetate (eta) fraction of O. aucheri; G, Michaelis-Menten curve of iodoacetamide as a standard.
4.3. The Kinetics of Protease Inhibition of Samples
The IC50 concentrations of the samples were 0.002 - 0.46 mg/mL based on Table 1. Table 1 shows the Km and Vmax of the samples against protease. Compared to the control without inhibitor, the Vmax reduction was significant for all the samples except the petr fraction (P < 0.05). The Km was decreased for all the samples except for the petr fraction of O. aucheri, in which Km = 0.03 ± 0.003, remained constant (Table 1, P < 0.05). The types of protease inhibition by all the samples and by iodoacetamide as a positive standard were uncompetitive (Table 1), except for the petr fraction of O. aucheri, which exhibited mixed inhibition (Table 1).
5. Discussion
Coronaviruses can cause infections in the respiratory tract, such as SARS and MERS, in different animals and humans (17, 19, 20). The consumption of natural compounds, including antioxidant agents to control the immune system, can be useful for afflicted patients. Throughout human history, many epidemic disorders have appeared. Ibn Sina’s Canon of Medicine (21) and other physicians proposed solutions to these epidemics that were effective in controlling these diseases. Herbal recommendations (use of plants, extracts, and compounds) for the treatment of COVID-19 symptoms are according to traditional Persian medicine (21). As a result, it is recommended that these patients use natural compounds such as flavonoids and tannins (22) as anti-inflammatory and antioxidant factors to regulate their immune systems. Therefore, the utilization of natural compounds, including flavonoids and tannins (22) as anti-inflammatory and antioxidant factors, to regulate the immune system, is suggested for these patients (23-26). The herbal components, especially terpenoids, possess protease inhibition activities, so these may be potentially effective due to low IC50 values for the management of viral epidemics. These components can be efficiently utilized as antiprotease drugs for SARS-CoV-2 in the future (27).
Otostegia aucheri Boiss is endemic to Pakistan and Iran. This genus includes eight species that are commonly scattered in the Mediterranean and southwestern Asia (28, 29). Phenolic compounds are recognized in Otostegia species. These compounds are well-known for their anti-inflammatory and antibacterial activities (30). Apigenin in O. aucheri may contribute to the inhibition of the enzyme (15). The results of this study showed that the IC50 of O. persica extract was 0.46 ± 0.005 mg/mL (Table 1). On the other hand, different fractions of O. persica had no significant inhibitory effects on the inhibition of protease. Also, the IC50 of buta and chlo fractions of O. aucheri were 0.07 ± 0.002 mg/mL and 0.09 mg/mL, respectively (Table 1), and the IC50 of iodoacetamide as the standard was 0.01 ± 0.002 mg/mL (Table 1). The eta fraction of O. aucheri has the greatest inhibition of protease activity (IC50 = 0.002 ± 0.0001), which is more than iodoacetamide (IC50 = 0.01 ± 0.002 mg/mL) as a standard (Table 1, P = 0.04). The IC50 values of petr, chlo, and buta fractions of O. aucheri were significantly different in comparison to iodoacetamide (P = 0.037). Also, in other research, it is reported that the eta fraction of O. persica (30) had the most α-amylase inhibitory activity, which was similar to acarbose used as a standard (30).
For the first time (Table 1), the mode of protease inhibition of O. persica, O. aucheri extracts, and their different fractions were evaluated, and the results showed that all of the samples, except for the petr fraction of O. aucheri, inhibited protease through an uncompetitive mechanism. Also, iodoacetamide, as a positive standard, inhibited protease through an uncompetitive mechanism in which the inhibitor binds to the enzyme-substrate complex, reducing both Vmax and Km (Table 1). The petr fraction of O. aucheri inhibited protease through a mixed mechanism (Table 1). One advantage of uncompetitive and mixed inhibitors is that they would be effective at lower concentrations of substrate (9); in other words, their inhibitory power is not affected by the concentration of the substrate (8). The comparison of Km and Vmax in the presence and absence of inhibitors showed the mode of retardation. If the value of Vmax is fixed and that of Km increases, the inhibition is competitive, and if the value of Vmax is reduced and that of Km is constant, the inhibition is noncompetitive. If the values of Vmax and Km are reduced, the inhibition is uncompetitive; otherwise, the inhibition is mixed (8). It was reported that the methanol extracts of Salvia macilenta and Salvia officinalis revealed uncompetitive inhibition on alpha-glucosidase as a marker enzyme of diabetes (31). In another research, anti-protease activities from different parts of Salvia aculeatissimum fruits were examined, and Dixon plots and Lineweaver–Burk double reciprocal plots revealed a competitive mode of inhibition (32).
5.1. Conclusions
The genus Otostegia is a significant source of biologically active natural compounds and active secondary metabolites. The crude extracts and fractions of different plants belonging to the Lamiaceae family contain good anti-inflammatory and antimicrobial factors (7). Different fractions of O. aucheri can significantly inhibit shrimp protease as a 3CLpro model. The eta fraction of O. aucheri, with an IC50 = 0.002 mg/mL compared to iodoacetamide at 0.01 mg/mL, showed the highest protease inhibition, although different fractions of O. persica did not significantly inhibit protease activity. This difference may be attributed to the flavonoids and terpenoids in the O. persica and O. aucheri extracts. Molecular docking studies could identify active compounds, and HPLC-MS could identify active flavonoids. In addition, the purification of active compounds and clinical trials are warranted to validate therapeutic potential, pending in vivo pharmacokinetic studies.
5.2. Limitations
This study has five limitations. First, the fractionation of ethanol extracts of the studied plants was only performed with petr, chlo, buta, and eta. Future studies should compare different methods of extraction, such as the Soxhlet method, which is a common method for the extraction of lipids, sterols, and fatty acids (33), and fractionation. Second, the experiments were only performed on crude extracts and different fractions of O. persica and O. aucheri, not on purified compounds of these two plants. Future studies should purify the active compounds of these two plants and evaluate protease inhibition and the mode of inhibition. Also, differences in harvest time or location may affect flavonoid and terpenoid content. Third, in vitro results may not fully reflect in vivo efficacy due to potential metabolism in vivo. Thus, animal or clinical studies are needed to confirm protease inhibition efficacy. Fourth, shrimp protease may not fully mimic 3CLpro kinetics and may differ in the active site from 3CLpro. Fifth, there is a limitation on assay specificity as casein may not mimic viral polyproteins.