1. Background
Nowadays, insomnia is a widespread health complaint and a common problem or disease in our restless society. Approximately 10 to 20 percent of adults suffer from chronic insomnia (1). Insomnia is often defined by sleeping disorders. People with insomnia may encounter difficulty initiating sleep, remaining asleep during the night, or achieving adequate sleep quality to some degree (2). Insomnia can be caused by psychiatric disorders, physical conditions like cardiopulmonary failure and chronic pain, and disruptions in sleep-wake patterns. Substances like caffeine, nicotine, alcohol, and amphetamines may also contribute to the development of insomnia (3). In patients with and without a history of psychiatric disorders, the presence of insomnia can increase the likelihood of developing or experiencing a relapse of depression and anxiety. Therefore, the detection and treatment of patients who suffer from insomnia might have important preventative value against developing mental disorders (4). Insomnia can be treated by synthetic medicines such as benzodiazepines and newer non-benzodiazepines, which have a number of side effects (fatigue, cognitive impairment, and physical dependence) (5). In recent years, herbal sleep aids have emerged as an alternative or complementary medicine to improve sleep quality and lessen side effects (6).
Lactuca sativa L. (garden lettuce), belonging to the Asteraceae family, is a leafy annual herb used for salads in Iran. The seeds of lettuce have traditionally been used as a sleeping aid and for relieving inflammation and osteodynia (7). In Persian medicine, the hypnotic effects of lettuce have been mentioned, and it has been introduced as one of the most effective sleep-inducing herbs (8).
There are some reports about the antioxidant, anti-inflammatory, analgesic, neuroprotective, and sedative-hypnotic activities of lettuce (7-11). In one study, the sedative effect of L. sativa seed oil was investigated in sixty patients suffering from insomnia. The results of this investigation indicated that this plant is a safe and effective sleeping aid (12).
2. Objectives
The objective of the research was to investigate the hypnotic activity of L. sativa seed syrup in a double-blind, randomized clinical trial.
3. Methods
3.1. Preparation of Syrup and Placebo
Lettuce seeds were obtained from an Iranian herbal shop. The seeds (24,000 g) were soaked in water for 24 hours at room temperature and then extracted by the decoction method for 30 minutes. The extract was filtered with Whatman cellulose filter papers. The extract was concentrated using the Bain-Marie method. The yield of the aqueous extract of L. sativa seed was 7.56%. The color of the extract was light to dark brown with a mild herbal scent and natural herbal flavor. To produce the syrup, sucrose (80%) was combined with the extract. The concentration of the lettuce syrup was 6.67% w/v. The placebo was prepared according to the pharmacopoeia Simple syrup formula. Permitted coloring agents were added to the placebo to make it look the same as the lettuce seed syrup. The drug or placebo was provided in 120-milliliter bottles and administered to patients.
Both the lettuce seed syrup and placebo had the same appearance, packaging, and labeling and were distributed between groups 1 and 2. The syrup was prescribed to the patients by a physician based on the number of labels. Blinding was maintained for both the prescribing physician and the person presenting the lettuce seed syrup or placebo. Only the pharmacist knew which numbers corresponded to the lettuce seed syrup or the placebo.
3.2. Measurement of Total Phenolic Contents
To measure the total phenol content, the Folin-Ciocalteu method was utilized (13). One milliliter of L. sativa extract was combined with 5 mL of Folin-Ciocalteu reagent, which was diluted 10 times with distilled water prior to use. After 10 minutes, a 4 mL volume of sodium bicarbonate solution (75 g/L) was added to the mixture and left to stand at room temperature for 30 minutes. Absorbance was then recorded at 765 nm using a UV spectrophotometer (Pharmacia Biotech). The phenolic content was determined using a calibration curve generated from the absorbance values of gallic acid (GA) standards ranging from 75 to 200 mg/L. Results are reported as milligrams of gallic acid equivalents (GAEs) per gram of dry extract (13, 14).
3.3. Sleep Study Design
This investigation was a prospective double-blind randomized study and a 4-week clinical trial performed at Golestan Hospital, Ahvaz, Iran. This research received approval from the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences. Furthermore, the trial was registered with the Iranian Registry of Clinical Trials under the registration number IR.AJUMS.REC.1398.424. Primary insomnia is sleeplessness that is not due to psychiatric disorders, environmental factors, drugs, or foods. The criteria for diagnosing primary insomnia, as defined by the diagnostic and statistical manual of mental disorders, fourth edition, text revision (DSM-IV-TR), are mentioned in the following (15).
The symptoms of primary insomnia according to the DSM-IV-TR are trouble falling asleep, staying asleep, or feeling unrefreshed after sleep, and these issues must persist for at least one month. The sleep disturbance causes impairment in social or occupational functions, and the sleep problems do not occur exclusively during the course of a mental, neurological, or other medical sleep disorder (15).
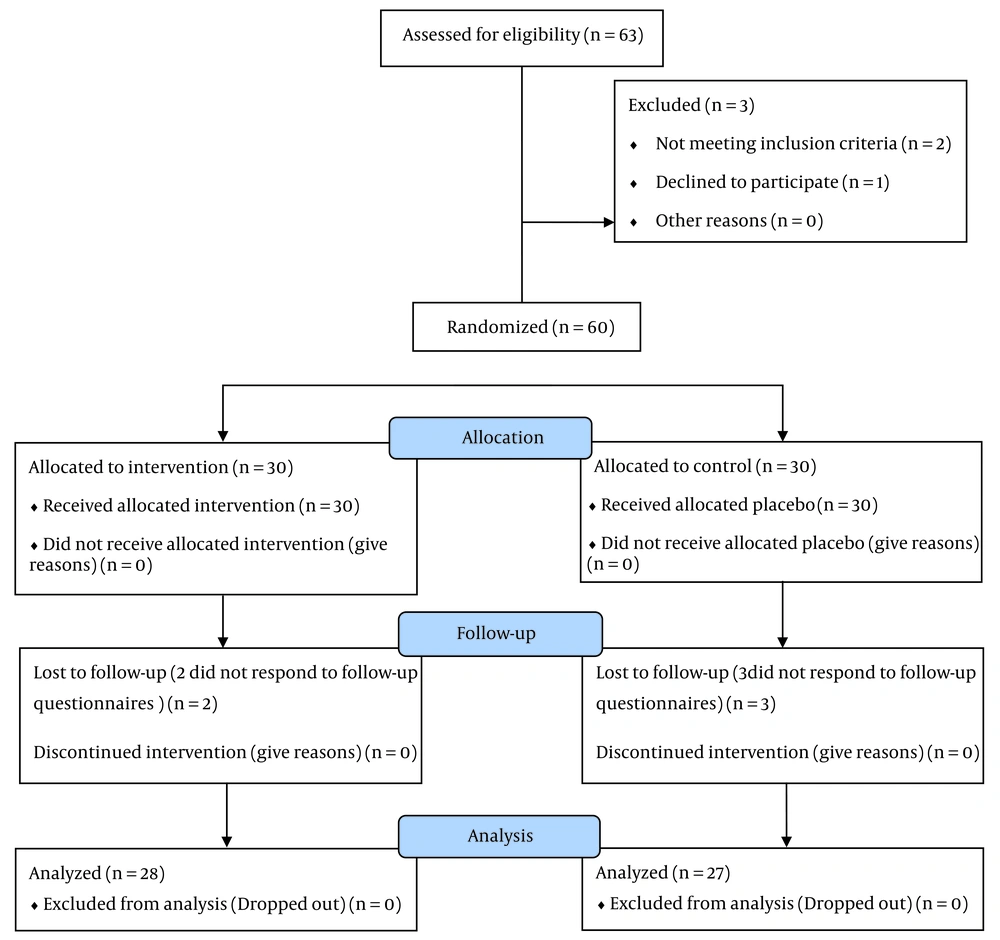
Sixty patients (women and men) between the ages of 20 and 50 with a definite diagnosis of primary insomnia according to the DSM-IV-TR, following a thorough review of the medical history and completion of a physical examination, were enrolled. Patients with secondary insomnia (a sign or consequence resulting from another underlying issue, such as an emotional, psychiatric (such as bipolar or depressive illness), neurological, or other medical sleep disorder), severe heart, renal, or liver dysfunction, concomitant organic or neuropsychiatric disease, and pregnancy were excluded. Patients who were using any other medicinal herbs that might influence the study outcome were also excluded. Participants were randomly assigned into two groups. In the study, participants were randomly assigned to either the lettuce group or placebo group in a 1:1 allocation ratio. Group 1 (n = 30) received 15 ml of lettuce seed syrup every night for 4 weeks, and group 2 (n = 30) was given 15 ml of a matching placebo. Randomization was performed using a software-generated block randomization method. The study flowchart is shown in Figure 1. All participants signed a written informed consent before entering the study.
3.4. Subjective Data
Sleep quality was measured by the subjective rating of the Pittsburgh Sleep Quality Index (PSQI), a self-noted questionnaire assessing subjective sleep quality over a one-month period. The PSQI was assessed at both the beginning and the conclusion of the study. This 19-item self-administered questionnaire evaluates sleep over a one-month period and generates seven component scores: Subjective sleep quality, time taken to fall asleep (sleep latency), total sleep duration, habitual sleep efficiency, frequency of sleep disturbances, use of sleep medication, and level of daytime dysfunction over a one-month period. The results of the PSQI show a total score reflecting subjective sleep quality (0 - 21), with higher scores reflecting poorer sleep quality (poor sleepers: PSQI > 5 and good sleepers: PSQI ≤ 5) (16, 17).
3.5. Statistical Analysis
The data are reported as mean ± standard deviation (SD). To evaluate differences in mean scores within each group before and after the intervention, a paired sample t-test was conducted. Statistical significance was considered at P < 0.05, and SPSS 22 was utilized.
4. Results and Discussion
The results showed that the total phenolic content of L. sativa seed syrup, in reference to the standard curve for GA (y = 0.0074x - 0.0756, R2 = 0.9858), was 111.16 mg GAE per 1 g dry extract. Lettuce seed syrup is standardized based on total phenols (Folin-Ciocalteu method) content. Each 15 mL of syrup contains 1 g aqueous extracts of L. sativa containing 111.16 mg total phenols as GAEs. In this study, the hypnotic effect of L. sativa seed syrup in patients with insomnia was investigated. Table 1 presents the baseline characteristics of the study population, with no significant differences observed between the groups.
| Variables | Case Group | Control Group | P-Value |
|---|---|---|---|
| Age (y) b | 34.43 ± 9.80 | 36.33 ± 9.55 | 0.450 |
| Gender | 0.399 | ||
| Male | 8 | 10 | |
| Female | 22 | 20 | |
| Known sleep disorder (OSA, restless legs, etc.) | 0 | 0 | - |
| Ethanol (g/day) | 0 | 0 | - |
a Values are expressed as mean ± standard deviation (SD).
b Age range: 20 - 50 y.
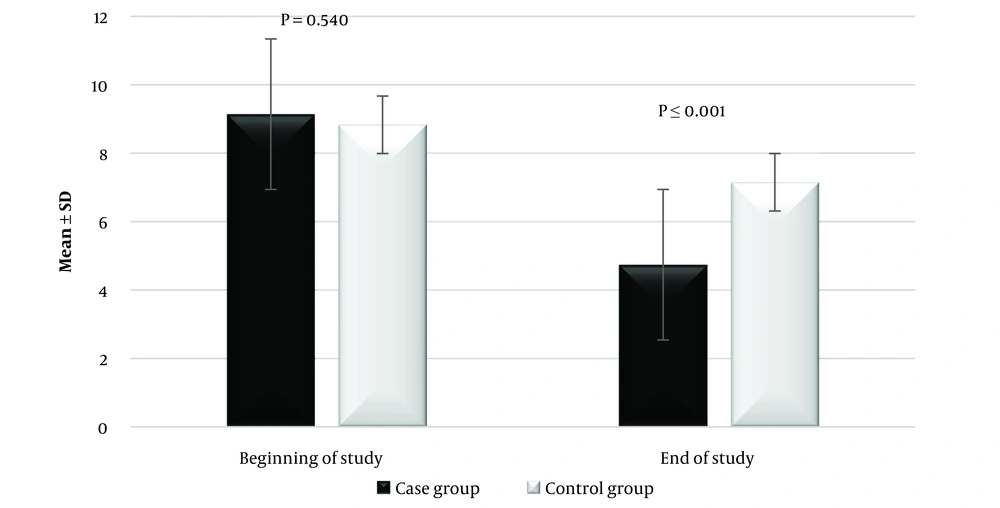
Two participants did not respond to the PSQI questionnaires performed one month after receiving lettuce seed syrup. The remaining 28 participants completed the one-month study for sleep improvement and responded to the PSQI questionnaires. In the control group, 3 participants did not respond to follow-up questionnaires. Ultimately, 28 patients in the treatment group and 27 patients in the placebo group were considered in the final analysis. At the start of the study, there was no significant difference in PSQI scores between the case and control groups. Figure 2 shows the comparison of the global sleep quality of the case and control groups at the beginning and end of the study. At the start of the study, there was no significant difference in global sleep quality between the case and control groups (P = 0.540).
In the case group, the mean global score of the PSQI was 9.14 ± 2.13 at the beginning of the investigation (range: 7 - 15). After 4 weeks of lettuce seed syrup treatment, it was reduced to 4.74 ± 1.58 (range: 1 - 8). After the intervention, in comparison to the control group, the change in PSQI score was significant for patients (P ≤ 0.001). The global PSQI score was 5 or less in 21 of the patients, 6 in three patients, 7 in two patients, and 8 in two patients. During treatment, no complaints or side effects were reported.
In the control group, the mean global score of the PSQI was 8.83 ± 1.67 at the beginning of the study. After one month of placebo treatment, it was reduced to 7.15 ± 2.18. At the end of the study, the change in PSQI score was significant in the control group compared to baseline (P ≤ 0.001). The mean and SD of each component score for the case and control groups at the beginning and end of the study are shown in Table 2. Compared to the control group, the changes in the mean global score of the PSQI in the case group were significant at the end of the study (P ≤ 0.001).
| Variables | Case Group | Control Group | ||||
|---|---|---|---|---|---|---|
| Beginning of Study | End of Study | P-Value b | Beginning of Study | End of Study | P-Value | |
| Subjective sleep quality | 2 ± 0.65 | 1.31 ± 0.66 | ≤ 0.001 | 1.83 ± 0.85 | 1.70 ± 0.67 | 0.265 |
| Sleep latency | 2.41 ± 0.82 | 1.59 ± 0.63 | ≤ 0.001 | 2.48 ± 0.74 | 2.14 ± 0.72 | 0.233 |
| Sleep duration | 1.07 ± 0.10 | 0.72 ± 0.80 | 0.002 | 1.10 ± 0.62 | 0.81 ± 0.56 | 0.022 b |
| Habitual sleep efficiency | 0.62 ± 0.82 | 0.10 ± 0.31 | ≤ 0.001 | 0.38 ± 0.68 | 0.30 ± 1.60 | 0.814 |
| Sleep disturbances | 0.59 ± 0.68 | 0.34 ± 0.55 | 0.032 | 0.59 ± 0.68 | 0.40 ± 0.64 | 0.031 b |
| Use of sleeping medication | 1.83 ± 1.17 | 0.52 ± 0.63 | ≤ 0.001 | 1.72 ± 1.16 | 1.59 ± 1.05 | 0.022 b |
| Daytime dysfunction | 0.38 ± 0.56 | 0.10 ± 0.31 | 0.009 | 0.45 ± 0.63 | 0.18 ± 0.40 | 0.017 b |
| Global sleep quality | 9.14 ± 2.13 | 4.74 ± 1.58 | ≤ 0.001 | 8.83 ± 1.67 | 7.15 ± 2.18 | ≤ 0.001 b |
a Values are expressed as mean ± standard deviation (SD).
b Significant differences (P < 0.05).
The significant 53.08% reduction in average global PSQI score after four weeks, along with improvements in the sleep quality category, suggests that lettuce seed syrup had a clear positive effect. The patients treated with L. sativa seed showed significantly greater improvement in Sleep Rating Scale scores compared to patients receiving placebo (P < 0.05). No apparent side effects were observed to be attributable to L. sativa at the used dosage. In the control group, the reduction in average global PSQI score after four weeks can be attributed to the placebo effect.
The placebo effect can be triggered through verbal suggestions and conditioning, as well as by past experiences that influence a patient’s expectations. Numerous studies have shown that the placebo effect plays a significant role in health outcomes for certain conditions. Diseases such as migraines, joint pain, arthritis, asthma, high blood pressure, and depression are particularly responsive to the placebo effect. It is a multifaceted phenomenon, involving various psychological and neurobiological processes (18, 19).
Lactuca sativa contains phenolic compounds and flavonoids like flavonol glycoside (lactucasativoside A, japonicin A, quercetin, isoquercitrin), caftaric acid, and chlorogenic acid (20-). The sedative effects of herbal medicines have been linked to various phytochemical compounds, including flavonoids, terpenoids, and saponins (23).
Flavonoids may have a calming effect that encourages sleep by interacting with the brain’s GABA-A receptors. This mechanism is believed to resemble the action of benzodiazepines, with flavonoids acting as positive allosteric modulators on these receptors. In particular, certain flavonoids bind to GABA-A receptors, amplifying the effects of GABA, a neurotransmitter that slows down nerve activity and induces relaxation and sleep (24-27).
In one previous study, the sedative and hypnotic effect of L. sativa oil in patients suffering from sleep disorders was investigated. As a result, soft gelatin capsules containing 1000 mg of purified oil from L. sativa seeds significantly enhanced sleep scores without causing any side effects (11). Different limitations may have affected the findings of the present study, one of which was the low number of patients and the difficulty in generalizing the results. Therefore, these findings should be validated through a larger, randomized controlled study. Another limitation is the unpleasant flavor of L. sativa seed syrup, and it is proposed to study the effect of lettuce seed, leaf, or stem in other dosage forms like capsules. Additionally, it is suggested to investigate the hypnotic activity of isolated compounds from lettuce in clinical trials. The authors investigated the effect of lettuce seed on primary insomnia and propose studying the efficacy of treatment on secondary insomnia.
In this clinical trial, sleep quality was measured by the subjective rating of the PSQI, which is a self-noted questionnaire. Some participants did not respond to the PSQI questionnaires honestly. Additionally, some patients did not complete the study, with reasons for discontinuing including failure to follow up or noncompliance. The use of complementary and alternative medicine has been increasing globally, yet a gap remains between alternative medicine and evidence-based medicine, so the evaluation of the safety and efficacy of traditional herbal remedies is required seriously. Traditional herbal medicines are available in various over-the-counter dietary supplements, highlighting the need for further evaluation and clinical trials of these herbs in human subjects.
The research results should be made available for use in new industrial formulations. In conclusion, syrup of L. sativa seeds was found to be an effective sleeping aid. According to this study, lettuce seed syrup may be a hazard-free line of treatment. Larger randomized controlled investigations with a follow-up period after treatment are needed to confirm these results.