1. Background
Gastric ulceration is an injury to the mucosal epithelium caused by excessive secretion of gastric acid and aggressive pepsin activity (1). It is the most common gastrointestinal disorder, with treatment costs amounting to $4 billion (2). Helicobacter pylori infection, hemorrhagic shock, smoking, steroidal and nonsteroidal anti-inflammatory drugs (NSAIDs), and immobility are among the most reported aggressive factors contributing to the development of ulcers in the gastrointestinal tract (2). Indomethacin, an important member of the NSAID family, is used to reduce inflammation, pain, and fever (3). Indomethacin can cause stomach ulceration by increasing the acidity of gastric content and pepsin activity, while decreasing the mucus layer and bicarbonate secretion, leading to increased lipid peroxidation and reactive oxygen species (ROS) formation in the gastric mucosa (4). Despite the availability of synthetic antiulcer drugs, such as prostaglandin analogs, H2 receptor antagonists, and antacids, to manage and cure NSAID-induced gastric ulcers, gastrointestinal toxicity remains a significant obstacle to their clinical application. These drugs can cause mild to severe side effects and cannot prevent ulcer recurrence. Consequently, it is essential to investigate non-toxic, accessible, and cost-effective antiulcer medications (5).
Probiotics are characterized as live microbial dietary supplements that promote host health when administered in appropriate amounts (6). Several studies have suggested that probiotics have therapeutic and preventive effects in gastrointestinal diseases, including colic, acute infectious diarrhea, inflammatory bowel syndrome, antibiotic-associated diarrhea, travelers’ diarrhea, and lactose malabsorption (7). However, existing data on the potential relationship between probiotic administration and the healing and prevention of gastric ulcers are limited. Lactobacillus casei, found in high quantities in various fermented products like yogurt and cheese, has recently been shown to have a diverse array of biological functions, including gastroprotective properties, anticancer effects, and the ability to modulate immune responses (8). This probiotic has antipathogenic properties that can inhibit bacterial (e.g., H. pylori, Escherichia coli) and viral (e.g., herpes simplex virus, influenza virus) infections (9).
2. Objectives
Given the remarkable attributes of probiotics in alleviating gastrointestinal diseases, the present study was conducted to assess the antiulcerogenic activity of an oral powder containing metabolites of L. casei. The therapeutic efficacy of this formulation was compared to that of a reference drug, ranitidine, using an indomethacin-induced gastric ulcer model.
3. Methods
3.1. Microorganisms and Microbial Culture
The probiotic strain used was L. casei (PTCC: 39392), obtained from the Persian Type Culture Collection 125 (PTCC, Tehran, Iran). The bacteria were cultivated in MRS broth at 37°C for 24 hours and then maintained on MRS agar under anaerobic conditions (10).
3.2. Determining the Growth Rate of Probiotics in Culture Media
To estimate the growth rate of probiotics, 1 mL (equivalent to 103 cfu/mL) of freshly cultured L. casei was inoculated into 100 mL of MRS broth, the standard laboratory medium. After thorough mixing, the flask was incubated at 37°C for 36 hours, and the growth rate was determined by recording optical density (OD) every three hours (11).
3.3. Cell-Free Culture Supernatant Preparation
The L. casei strain was cultivated anaerobically in 100 mL of MRS broth at 37°C for 24 hours. Following this, the medium was centrifuged at 4000 rpm for 15 minutes at 20°C to obtain the supernatant. This supernatant, comprising a mixture of the culture medium and probiotic metabolites, was subsequently preserved at 4°C (10).
3.4. Study of Long-term Stability
The obtained supernatant was preserved at 4°C for 9 months, during which the lactic acid concentration was measured at regular intervals. Additionally, the pH was measured using a pH meter (Met Rohm®) throughout the storage period, and the findings were documented (10).
3.5. Formulation of a Stable Probiotic Powder
To enhance the stability and efficacy of the obtained probiotic supernatant, the freeze-drying method was employed. Specifically, the probiotic supernatant was freeze-dried at -50°C. After 48 hours, a dry, stable, and concentrated powder was obtained (12).
3.6. Animal Study
The study involved forty-two male Wistar rats, each weighing between 200 and 250 g. The animals were sourced from the Animal Center, Ahvaz Jundishapur University of Medical Sciences. The rats were kept separately in cages and maintained at a temperature of 25 ± 2°C, with a consistent 12-hour light and dark cycle. They had access to water and a pellet diet ad libitum. The Ethics Committee of Ahvaz Jundishapur University of Medical Sciences approved the study concerning the care and use of laboratory animals. The rats were given 1 hour to acclimate to their environment prior to the start of the experiments. After 48 hours of fasting, the rats were randomly divided into seven groups (n = 6) and administered orally as follows: Group 1: Indomethacin (30 mg/kg) suspended in carboxymethyl cellulose (CMC) (1%); group 2: Carboxymethyl cellulose (1%); groups 3, 4, and 5: Probiotic powder (60, 120, and 240 mg/kg, respectively) 30 minutes before the administration of indomethacin (30 mg/kg) suspended in CMC (1%); group 6: Probiotic powder (240 mg/kg); group 7: Ranitidine (50 mg/kg) 30 minutes before the administration of indomethacin (30 mg/kg) suspended in CMC (1%) (13).
3.6.1. Evaluation of Gastric Ulcers
After one hour, all animals were sedated using ketamine (50 mg/kg) and xylazine (10 mg/kg) before being sacrificed. Subsequently, their stomachs and greater curvature were dissected and washed with normal saline. The maximum length in mm of each lesion was measured under a dissecting microscope (ulcers measuring less than 1 mm were not scored). The total length of all lesions was defined as the Ulcer Index (14).
3.6.2. Histopathological Studies
The stomach tissue sections were fixed in a 10% buffered formalin solution, dehydrated, and subsequently immersed in paraffin. The paraffinized tissue sections were sliced to a thickness of 5 µm and stained with hematoxylin and eosin. All specimens were examined by a pathologist using a light microscope (Olympus DP12, U-TVO.5XC-2, Japan) at a magnification of 400 × (15).
3.6.3. Levels of Lipid Peroxidation
The content of malondialdehyde (MDA), which serves as an indicator of lipid peroxidation, was measured following a previously established protocol. Gastric tissues were homogenized in a phosphate buffer (pH = 7.4) and subsequently subjected to centrifugation. The upper layer was collected, and the intensity of the pink coloration was quantified by measuring the absorbance at 532 nm. The concentrations of MDA in the gastric tissue were determined using a standard curve and reported as nmol/g of wet tissue (16).
3.6.4. Evaluation of Interleukin-6 Concentration in Tissue
Concentrations of IL-6 in tissue were measured using commercially available ELISA kits according to the manufacturer’s protocol (17).
3.7. Statistical Analysis
The experimental findings are presented as the mean ± standard error of the mean (SEM). Upon verifying the normal distribution of the data, a one-way analysis of variance (ANOVA) and Tukey-Kramer’s post hoc test were performed using SPSS software (version 17.0). P-values below 0.05 were considered statistically significant.
4. Results
4.1. Growth Kinetics of Lactobacillus casei
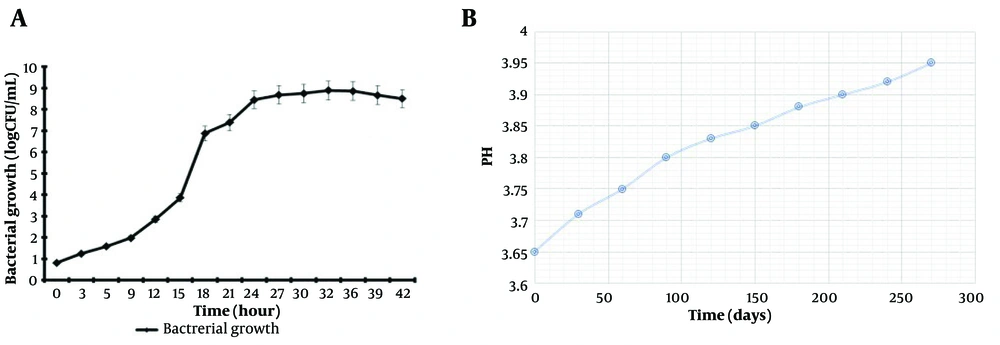
The plate-count method was used to estimate the approximate growth rate of L. casei in MRS medium. The growth curve of L. casei in MRS medium at 37°C for 42 hours under microaerophilic conditions is shown in Figure 1A. This figure indicates that L. casei increased from 102 to 109 after 24 hours.
4.2. Long-term Stability of Supernatant
Figure 1B shows that the pH of the probiotic supernatant increased from 3.65 to 3.95 over 9 months of storage at 4°C. Although these changes in pH were not statistically significant, even minor changes could affect the stability of the supernatant properties.
4.3. Animal Studies
4.3.1. Quantification of Ulceration
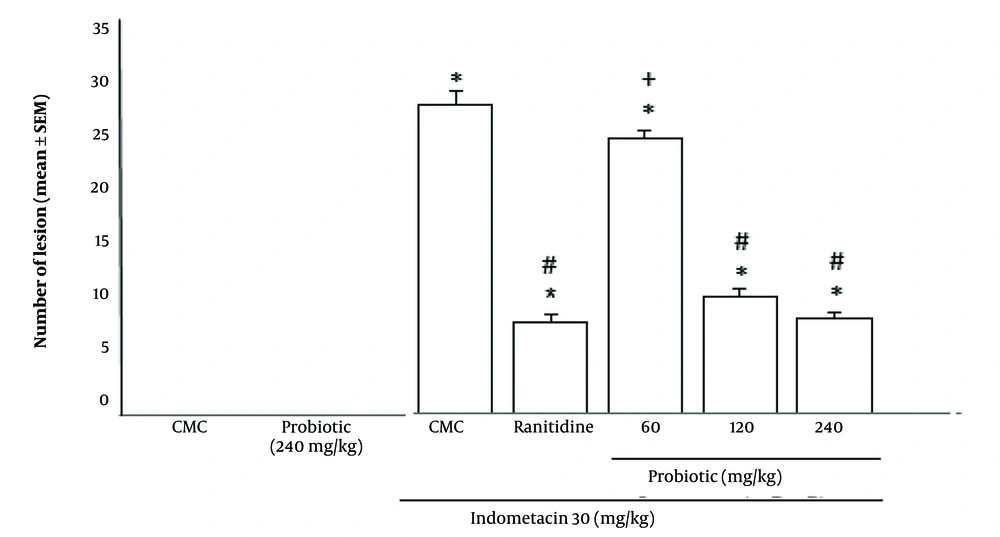
As shown in Figure 2, the mean number of gastric ulcers in the groups receiving probiotic powder (240 and 120 mg/kg) with indomethacin (30 mg/kg) was less than in the group receiving indomethacin alone (P < 0.05). There was no significant difference in the Ulcer Index between the groups receiving 240 and 120 mg/kg probiotic powder and the group receiving ranitidine (50 mg/kg). In contrast, administration of 60 mg/kg probiotic powder did not offer significant protection against ulceration compared to the standard drug (ranitidine) (P > 0.05).
Effect of oral powder containing Lactobacillus casei metabolites on Ulcer Index of indomethacin ulcerated rats. Data are expressed as means ± SEM; n = (6). All groups were compared to carboxymethyl cellulose (CMC) group (* P < 0.05), indomethacin plus CMC (# P < 0.05), ranitidine plus indomethacin (+ P < 0.05) according to analysis of variance (ANOVA) followed by Tukey post-hoc test.
4.3.2. Macroscopic Evaluation
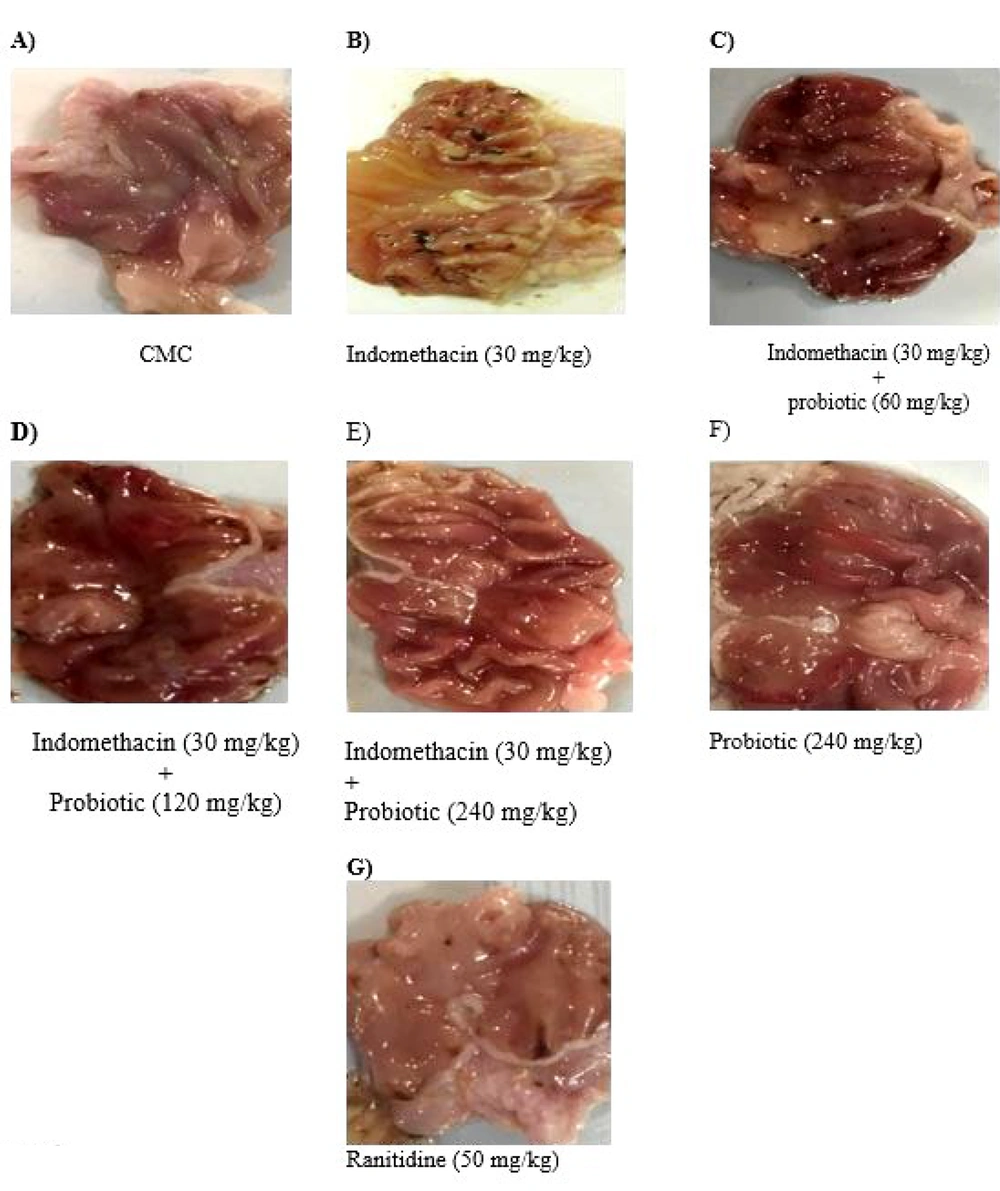
The animals receiving indomethacin (30 mg/kg) developed a consistent pattern of macroscopic damage, as evidenced by the presence of ulceration (Figure 3 B). No similar damage was macroscopically observed in the probiotic alone (240 mg/kg) and CMC alone groups (Figure 3A and F). Macroscopically, the administration of probiotic powder (60, 120, and 240 mg/kg) (Figure 3C, D , and E, respectively) and ranitidine (50 mg/kg) (Figure 3G) dramatically reduced the lesions observed in indomethacin-treated animals.
The gross appearance of gastric ulcers induced by Indomethacin(30 mg/kg) and protective effects of Powder Containing Probiotic Metabolites (60, 120, or 240 mg/kg). A, CMC; B, iandomethacin (30mg/kg); C, iandomethacin and pretreatment with 60 mg/kg Powder Containing Probiotic Metabolites; D, iandomethacin and pretreatment with 12o mg/kg Powder Containing Probiotic Metabolites; E, iandomethacin and Powder Containing Probiotic Metabolites (240mg/kg). F, powder Containing Probiotic Metabolites (240mg/kg); G, iandomethacin and ranitidine (50 mg/kg).
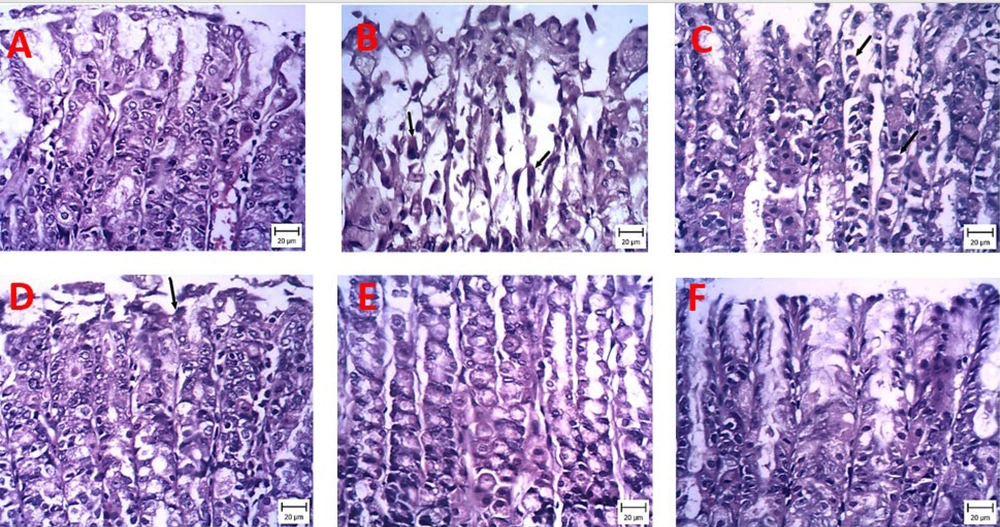
4.3.3. Histopathological Studies
Histopathological changes were investigated in the stomach sections of different treatment groups under a light microscope (Figure 4). H&E staining of sections from CMC rats showed well-preserved integrity of the mucous membrane, with tissues complete and clear, without specific damage (Figure 4A). In contrast, numerous lesions, coagulation necrosis, and edema below the mucosa with abundant empty spaces were observed in rats of the indomethacin (30 mg/kg) model group (Figure 4B). The 60 mg/kg probiotics group appeared very similar to the indomethacin group (Figure 4C). Although treatment with 120 mg/kg probiotics partially improved indomethacin-induced histopathological lesions, signs of damage remained (Figure 4D). As shown in Figure 4E, no significant damage was observed in the gastric mucosa of mice receiving 240 mg/kg probiotics, 50 mg/kg ranitidine, and CMC (Figure 4E).
Representative pictures of stomach sections stained with hematoxylin and eosin (H&E) examined under a microscope. Protective effects of powder containing probiotic metabolites (60, 120, or 240 mg/kg) on histopathological changes of gastric tissue induced by indomethacin (hematoxylin and eosin staining, magnification × 200). A, carboxymethyl cellulose (CMC); B, iandomethacin (30 mg/kg); C, iandomethacin and pretreatment with 60 mg/kg powder containing probiotic metabolites, D, iandomethacin and pretreatment with 120 mg/kg powder containing probiotic metabolites; E, iandomethacin and powder containing probiotic metabolites (240mg/kg); F, iandomethacin and ranitidine (50 mg/kg).
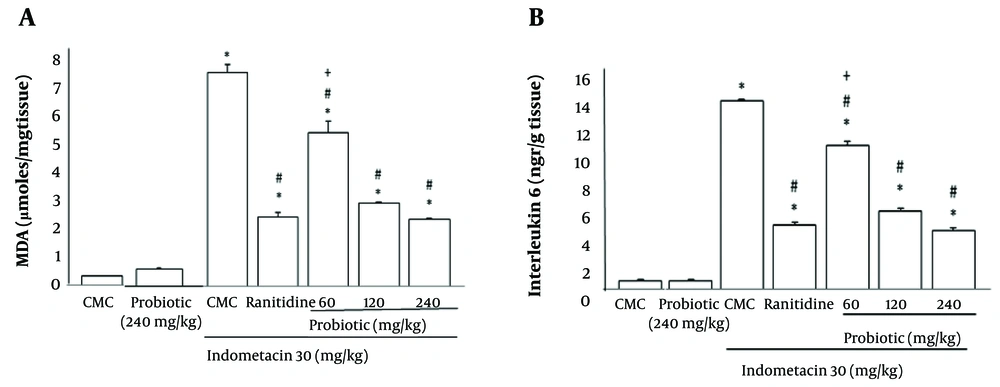
4.3.4. Levels of Lipid Peroxidation
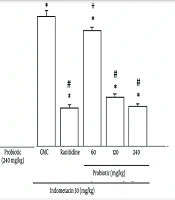
Figure 5A reveals the effects of oral powder containing L. casei metabolites on the lipid peroxidation of indomethacin-ulcerated rats. The MDA levels were significantly increased (P < 0.05) in the ulcerated animals. A significant reduction (P < 0.05) was observed in MDA levels in the probiotic plus indomethacin group (240 and 120 mg/kg) compared with the group receiving indomethacin plus CMC. These results were similar to those of the ranitidine plus indomethacin group. The 60 mg/kg probiotic group co-administered with indomethacin reduced lipid peroxidation compared to the indomethacin plus CMC-treated group, but this was not significant compared to the ranitidine plus indomethacin group.
A, Effect of oral powder containing Lactobacillus casei metabolites on levels of lipid peroxidation; B, effect of oral powder containing L. casei metabolites on interleukin-6 (IL-6) concentration in tissue. Data are expressed as means ± SEM; n = 6. All groups were compared to carboxymethyl cellulose (CMC) group (* P < 0.05), indomethacin plus CMC (# P < 0.05), ranitidine plus indomethacin (+ P < 0.05) according to analysis of variance (ANOVA) followed by Tukey post-hoc test.
4.3.5. Evaluation of Interleukin-6 Concentration in Tissue
As demonstrated in Figure 5B, probiotic treatment at all doses and ranitidine at 50 mg/kg induced a significant reduction in IL-6 levels compared to the indomethacin group (P < 0.05). Treatment with 60 mg/kg probiotics showed a remarkable alteration in this marker compared to the ranitidine group (P > 0.05).
5. Discussion
The NSAIDs have long been associated with the etiology of ulcers and have been utilized in various animal studies to induce gastric ulcers. In this study, indomethacin, a commonly used NSAID in animal experiments, was employed, and the results confirmed its effectiveness in inducing gastric ulcers (16). Indomethacin caused gastric ulcers, pathological lesions, and increased MDA and IL-6 levels in gastric tissue. Although acid inhibitors and antibiotics are commonly used to treat stomach ulcers, there is ample evidence of antibiotic resistance and side effects of these drugs, drawing more attention to the possible use of probiotics in the prevention and treatment of gastric ulcers (18). Recent experimental and clinical investigations have revealed that lactobacilli can be a potential treatment for gastric ulcers. Probiotics offer health benefits for gastric ulcers through mechanisms such as protecting the gastric mucosal barrier, enhancing prostaglandin production, increasing mucus secretion, stimulating growth factors and anti-inflammatory cytokines, improving the cell proliferation to apoptosis ratio, and promoting angiogenesis (18).
In this study, a rat model of indomethacin-induced gastric mucosal damage was utilized, revealing that oral administration of powder containing L. casei metabolites led to a decrease in mucosal damage, lipid peroxidation (measured by MDA content), and levels of the pro-inflammatory cytokine IL-6. Gastrointestinal mucus plays a crucial role in the primary defense mechanism against gastro-ulcerative factors. It aids in buffering gastric secretions and reduces erosion during stomach peristalsis and contractions. Administration of indomethacin to fasting rats caused damage to the gastric mucosa, resulting in an increased Ulcer Index. Pretreatment with oral probiotic powder (60, 120, and 240 mg/kg) and ranitidine (50 mg/kg) protected the gastric mucosa against indomethacin-induced gastric ulceration. The results of our histopathological studies also indicated the protective effects of oral probiotic powder (120 and 240 mg/kg). The 240 mg/kg probiotic powder-treated groups showed better histological architecture with fewer lesions, reduced coagulation necrosis, decreased edema, and comparatively a more compact mucosa. These findings are in accordance with earlier reports that probiotics improved macroscopic and microscopic gastric mucosal damage (19).
Gastric mucosal damage induced by indomethacin has been suggested to be mediated through enhanced oxidative stress (20). In the present study, indomethacin induced oxidative stress, as evidenced by a remarkable increase in lipid peroxidation level, expressed as MDA concentration. The MDA is a metabolite associated with oxidative stress, produced from unsaturated fatty acids via ROS-activated lipid peroxidation. Therefore, MDA is considered a biomarker for lipid peroxidation and is used to quantify and assess oxidative stress levels (19, 20). In this study, a marked increase in MDA level was observed in rats after indomethacin exposure. However, the administration of probiotics countered the rise in MDA levels. These results suggest that probiotics mainly alleviated indomethacin-induced gastric lesions by reducing oxidative stress.
In addition to the production of free radicals, a link between inflammation and indomethacin-stimulated gastric injury has been shown in various studies. Inflammation is a complex response of the host to tissue injury, characterized by increased secretion of pro-inflammatory cytokines like interleukin-1β (IL-1β) and IL-6 from immune cells (2). In this study, gastric mucosal levels of IL-6 were elevated in the indomethacin-induced gastric ulcer group, and probiotic pretreatment significantly reverted the levels of IL-6 to normal.
5.1. Conclusions
In conclusion, these results indicate that the mechanism by which probiotic metabolites ameliorate gastric ulcers might be associated with their antioxidant and anti-inflammatory effects through modulating the secretion of pro-inflammatory cytokines. According to these findings, the optimal dose for preventing gastric ulcers was found to be 120 mg/kg. The results suggest that oral administration of probiotic metabolites could be a promising supportive care for gastric ulcers. However, further studies on animal models and humans are needed to confirm this hypothesis. Additionally, long-term administration of oral dosage forms containing probiotic metabolites and their potential indications in human studies should be performed. Toxicity parameters must also be evaluated.