1. Background
Reactive oxygen species (ROS) can be produced by nicotinamide adenine dinucleotide phosphate (NADPH) (1, 2). Many different pathways in the body can generate ROS, such as in metabolic processes, xanthine oxidase (XO), endothelial dysfunction, and cyclooxygenase (COX) reactions (3). More importantly, the generation of ROS will lead to inflammation and is involved in some eye diseases. Because the eyes are always exposed to light, the visual system is highly susceptible to oxidative stress (3). Therefore, when ROS are produced beyond capacity, oxidative stress occurs, leading to eye diseases such as dry eye disease (DED), pterygium, cataracts, and many diseases of the cornea, optic nerve, and retina, age-related macular degeneration, retinopathy (3), and glaucoma (4). Therefore, the intracellular signaling pathway that generates ROS is closely related to vascular disorders that lead to the development of several eye diseases. Acacia pennata is one of the native acacia species grown in the Indochina region of Southeast Asia. It belongs to the thorny shrub category with a height of about 5 meters and grows wild throughout the area (5). Some regions have used its leaves as a substitute for vegetables. When mixed with milk, the juice of the leaves is used to treat child indigestion (5). Its roots are used to reduce flatulence and soothe stomach maladies. The bark of A. pennata can also be used to treat stomach ailments, as well as bronchitis and asthma (5). Acacia pennata is called 'rau gai thoi' and is widely distributed across Vietnam (6). Acacia pennata has been used as a traditional medicinal plant for various ailments such as cough, headache, rheumatism, and fever (5). Furthermore, A. pennata has been reported to have anti-inflammatory, antioxidant, antiparasitic properties, and transcriptional activity (7). Some other studies have also indicated that A. pennata contains compounds with the ability to inhibit the aggregation of β-amyloid and effectively support Alzheimer's disease (8). Recently, members of this research group have also demonstrated that A. pennata contains a compound that has anti-HIV-1 properties (6).
2. Objectives
However, the anti-inflammatory and ROS-inhibitory activity of the components in A. pennata remains unclear. Therefore, this study aimed to isolate the compounds with anti-inflammatory and ROS activities in A. pennata (L.) Willd.
3. Methods
3.1. Plant Material
Acacia pennata was selected from Son La province, Vietnam, in 2013. The voucher specimen was identified by the author (P. T. T.) with the code: SRD-01.2013. This sample is preserved at The National Institute of Medicinal Materials, Hanoi, Vietnam.
3.2. Chemicals
Lipopolysaccharide (LPS), dexamethasone (Dex), dimethyl sulfoxide (DMSO), and phospho-(Ser345)-p47phox were purchased from Sigma-Aldrich (St. Louis, MO). BD Pharmingen (Franklin Lakes, NJ) provided ELISA Test Kits for tumor necrosis factor (TNF)-α and interleukin (IL)-6. p47phox was obtained from Thermo Fisher Scientific. Silica gel column chromatography and RP-C18 column were purchased from Merck.
3.3. Analysis of the Structure
The Jasco P-1020 polarimeter was used to measure the optical rotation value [α]D. The Perkin-Elmer FTS Fourier Transform Infrared (FT-IR) spectrometer evaluated the infrared (IR) spectrum (KBr). The nuclear magnetic resonance (NMR) spectroscopy spectrum was measured using the FT-NMR Bruker AM500 device. The chemical shifts were recorded in parts per million in methanol-d4 (CD3OD) with tetramethylsilane (TMS). Electron emission ionization mass spectrometry (HR-ESI-MS) data were analyzed by the Thermo Scientific LTQ Orbitrap XL™.
3.4. Extraction and Isolation
The dried stems of A. pennata (7000 g) were cut into small pieces, soaked, and extracted with 96% ethanol at room temperature (3 times, each time for 4 days). The extract was combined with solvents and removed under reduced pressure to obtain a concentrated extract that was then dried (158 g). The concentrated extract was dissolved in distilled water (1.0 liter) to form a suspension, shaken, and then sequentially partitioned with n-hexane, ethyl acetate (EtOAc), and n-butanol. The n-hexane, EtOAc, and n-butanol extracts were separated, and the solvent was removed under reduced pressure to obtain the respective fractions. The n-hexane fraction yielded 14 g, the EtOAc fraction yielded 36 g, and the n-butanol fraction yielded 20 g. The EtOAc fraction (36 g) was applied to a column with silica gel. Then, the CH2Cl2-MeOH solvent system with an increasing methanol ratio from 0 to 100% was used for elution, yielding 5 fractions (F1 - F5).
The fraction F3 was further separated using a column with silica gel and an elution system of CH2Cl2-MeOH (15:1; 10:1; 8:1), yielding 5 fractions (F3.1 to F3.5). Fraction 3.2 was applied to the RP-18 silica gel column under reverse phase and eluted with a gradient using a MeOH/H2O solvent system (1/1; 2/1; 3/1), yielding compound number 1 (14 mg). Fraction 3.4 was separated on the RP-18 silica gel column under reverse phase and eluted with a solvent system of MeOH/H2O (1/2; 1/1; 2/1), yielding 4 fractions (F3.4.1 to F3.4.4). Fraction 3.4.3 was purified on the RP-18 silica gel column under reverse phase and eluted isocratically with a MeOH/H2O solvent system (3/2), yielding compound 3 3 (ARg1) (22 mg).
3.5. Macrophage Culture
Raw 264.7 was provided by the American Type Culture Collection (ATCC) and cultured according to the biobank instructions.
3.6. Determination of Cell Viability
Cell Counting Kit-8 (CCK-8, Japan) was used to evaluate the cytotoxicity affecting the cells.
3.7. Enzyme-Linked Immunosorbent Assay
The cytokine concentrations of TNF-α and IL-6 in the cells were analyzed using ELISA.
3.8. Western Blotting Analysis
Western blotting was performed as described (9). Antibodies (Abs) against phospho-(Ser345)-p47phox and p47phox were diluted 1:1000. Western blotting membranes were developed by chemiluminescence (ECL; Amersham-Pharmacia).
3.9. Reactive Oxygen Species Analysis
The analysis of ROS was performed (9). Cells were treated with or without ARg1 for 45 minutes. Then, LPS was added. The treated cells were incubated with DHE (Calbiochem, 15 min) at 37ºC in CO2. The relative fluorescence intensity of each group was analyzed using a fluorescence microscope with laser scanning (LSM 510) and Carl Zeiss (LSM510).
3.10. Determination of Nicotinamide Adenine Dinucleotide Phosphate Oxidase Activity
The activity of NADPH oxidase in cells was analyzed using the chemiluminescence Lucigenin assay as previously described (9).
3.11. Statistical Method
Mean ± standard deviation (SD) is shown from data of independent experiments and analyzed by the Student's t-test with ANOVA used for multiple comparisons. Statistical significance was considered to be different at P < 0.05.
4. Results
4.1. Structure of Compound 3
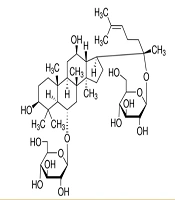
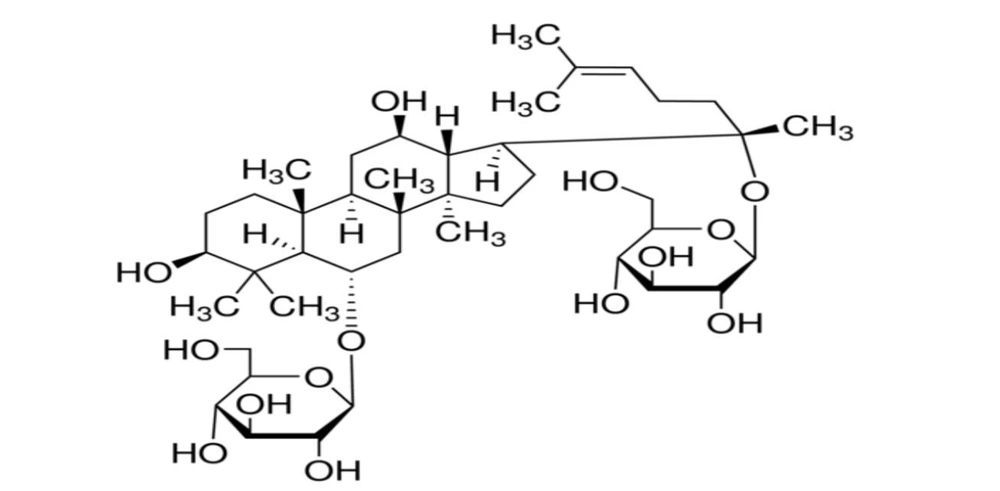
After purifying 3 ARg1, this substance was obtained in the form of a white powder. The 1H, 13C- NMR spectroscopy (Tables 1 and 2), distortionless enhancement by polarization transfer (DEPT), heteronuclear single quantum coherence (HSQC), and heteronuclear multiple bond correlation (HMBC) spectra of ARg1 indicate that this is a tetracyclic triterpenoid saponin with a dammarane skeleton, featuring two sugar moieties in its structure (10, 11). Both sugar moieties have a β configuration due to the two anomeric protons appearing as doublets at δH = 4.37 (H-1′), δH = 4.62 (H-1′′) with a coupling constant J = 7.5 Hz. The first sugar moiety was identified as attached to C-6 of the aglycon framework due to the interaction appearing at δH = 4.37 (H-1′), δC = 80.9 (C-6), while the other sugar moiety was determined to be attached to C-20 of the aglycon framework based on the interactions δH = 4.62 (H-1′′), δC = 84.9 (C-20) observed in the HMBC spectrum. The ESI-MS mass spectrum has an ion peak at m/z: 801 [M + H] + (positive), combined with the NMR spectral data, indicates that the molecular formula of ARg1 is C42H72O14 (M = 800) (Figure 1). Analyzing the spectral data and referring to the literature identifies ARg1 as ginsenoside Rg1. Ginsenoside Rg1 is the main component found in species belonging to the genus Panax (12).
| Position | 1H |
|---|---|
| 6 | 4.12 (1H, d, J = 3.0 Hz) |
| 18 | 1.12 (3H, s) |
| 19 | 1.02 (3H, s) |
| 21 | 1.37 (3H, s) |
| 24 | 5.13 (1H, t, J = 6.5 Hz) |
| 26 | 1.70 (3H, s) |
| 27 | 1.65 (3H, s) |
| 28 | 1.35 (3H, s) |
| 29 | 1.03 (3H, s) |
| 30 | 0.98 (3H, s) |
| 1′ | 4.37 (1H, d, J = 7.5 Hz) |
| 1′′ | 4.62 (1H, d, J = 7.5 Hz) |
| Position | 13C |
|---|---|
| 1 | 40.2 |
| 2 | 27.2 |
| 3 | 79.8 |
| 4 | 40.4 |
| 5 | 61.8 |
| 6 | 80.9 |
| 7 | 45.2 |
| 8 | 41.9 |
| 9 | 50.6 |
| 10 | 40.5 |
| 11 | 30.9 |
| 12 | 717 |
| 13 | 49.8 |
| 14 | 52.5 |
| 15 | 31.5 |
| 16 | 27.6 |
| 17 | 53.1 |
| 18 | 17.6 |
| 19 | 17.8 |
| 20 | 84.9 |
| 21 | 22.8 |
| 22 | 36.6 |
| 23 | 24.2 |
| 24 | 125.8 |
| 25 | 130.3 |
| 26 | 25.8 |
| 27 | 17.9 |
| 28 | 31.4 |
| 29 | 16.1 |
| 30 | 17.1 |
| 1′ | 105.6 |
| 2′ | 75.5 |
| 3′ | 79.1 |
| 4′ | 71.4 |
| 5′ | 77.7 |
| 6′ | 62.9 |
| 1′′ | 98.3 |
| 2′′ | 75.4 |
| 3′′ | 78.3 |
| 4′′ | 71.3 |
| 5′′ | 77.9 |
| 6′′ | 62.7 |
4.2. Cytotoxicity of Compound 3 to Cell Viability
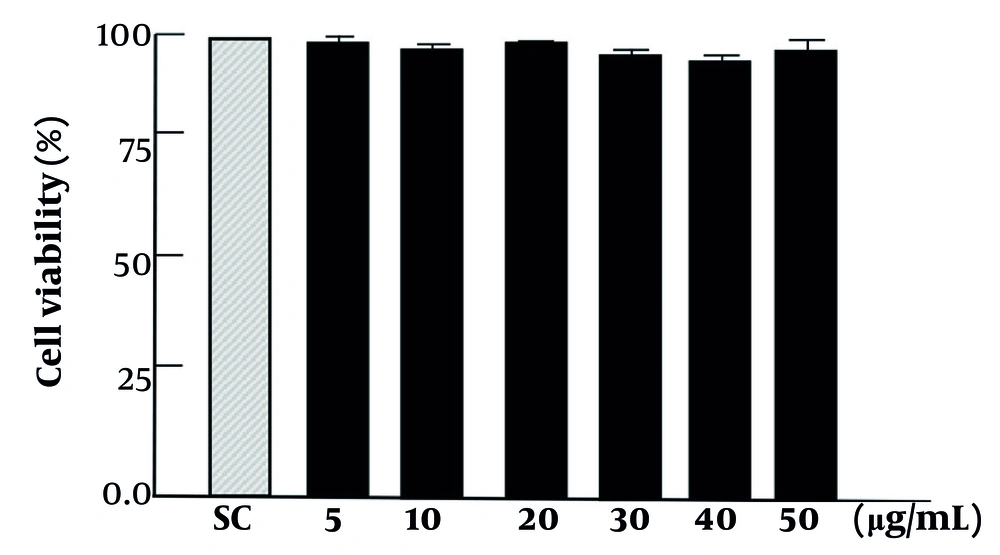
The concentration of 10 to 50 μg/mL of ARg1 does not affect the viability of the cells (Figure 2).
The activity of compound 3 (ARg1) does not affect the viability of the cells. The concentrations of ARg1 were incubated in wells with a density of 1 × 105 cells. Cell Counting Kit-8 (CCK-8) was used to assess the viability of the cell. Three independent experiments were shown as mean ± standard deviation (SD); SC, 10% dimethyl sulfoxide (DMSO).
4.3. Lipopolysaccharide-Activated Macrophage Lipopolysaccharide-Induced Cytokine Production in Macrophages Was Inhibited by Compound 3
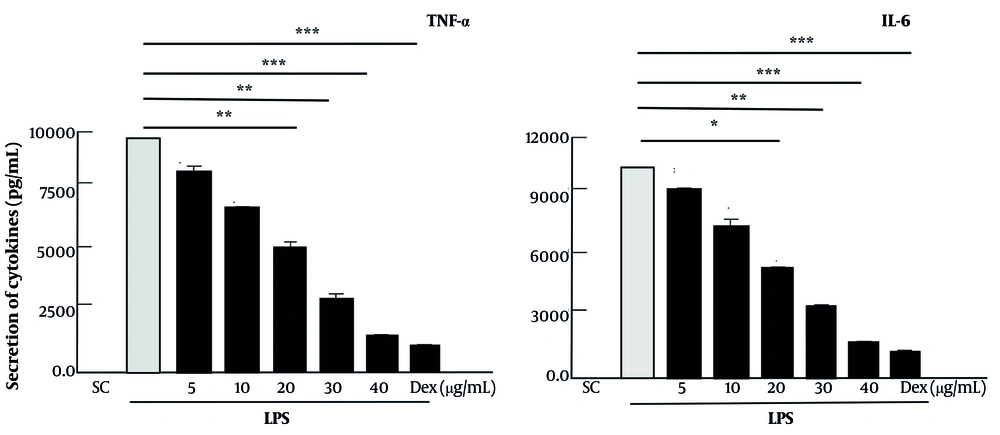
The anti-inflammatory effect of ARg1 was evaluated. The TNF-α and IL-6 in macrophages were decreased when cells were treated with ARg1 at a concentration of 40 μg/mL compared to the positive control (P < 0.001). Furthermore, ARg1 (40 μg/mL) strongly inhibited TNF-α and IL-6 nearly equivalent to treatment with Dex (a type of anti-inflammatory corticosteroid, Figure 3).
Pro-inflammatory cytokine induced by lipopolysaccharide (LPS)-stimulated inflammatory response has been inhibited by compound 3 (ARg1). Supernatant samples were collected after incubation with LPS (1 µg/mL) for 18 hours with cells that had been pre-incubated with ARg1 concentrations or dexamethasone (Dex) (1 µg/mL) for 45 min to evaluate cytokine. Five independent experiments were shown as mean ± standard deviation (SD). * P < 0.05, ** P < 0.01, *** P < 0.001 is a significant difference. SC, 10% dimethyl sulfoxide (DMSO).
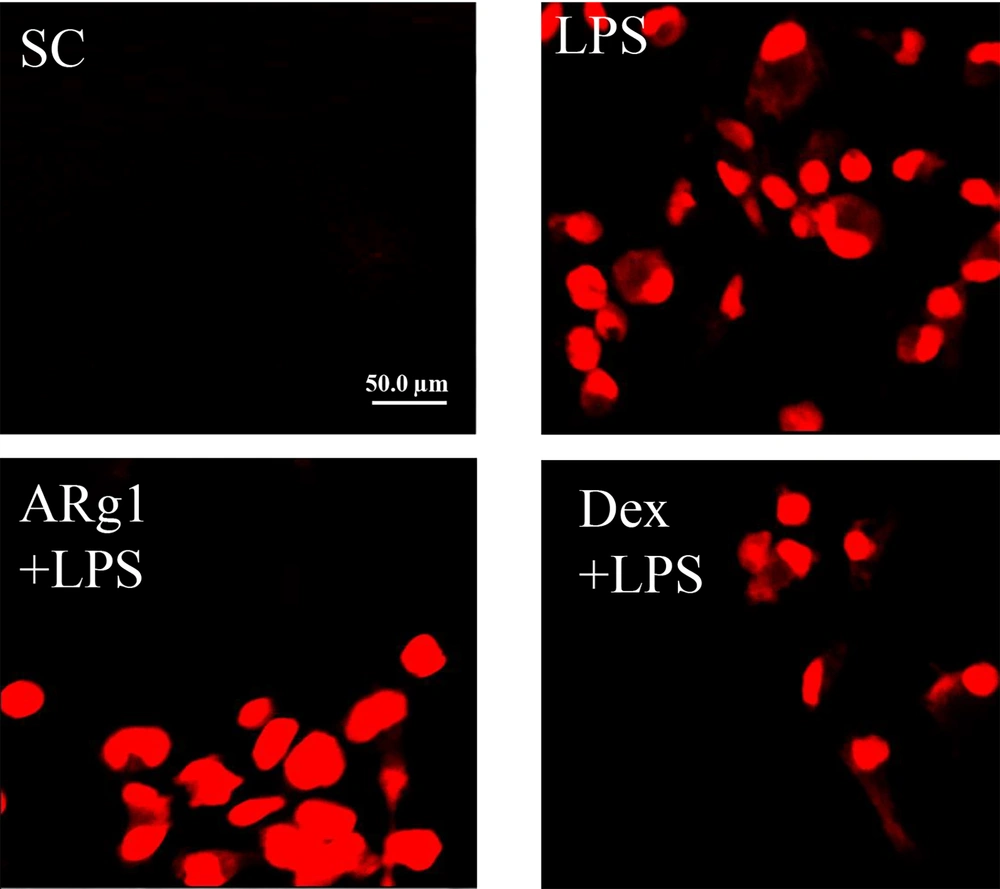
4.4. Compound 3 Inhibits Reactive Oxygen Species
The effect of ARg1 on LPS-mediated regulation of cellular ROS was evaluated. As shown in Figure 4, LPS strongly stimulated cellular ROS, but cellular ROS generation was inhibited in the presence of ARg1 or Dex compared to the positive control (40 μg/mL).
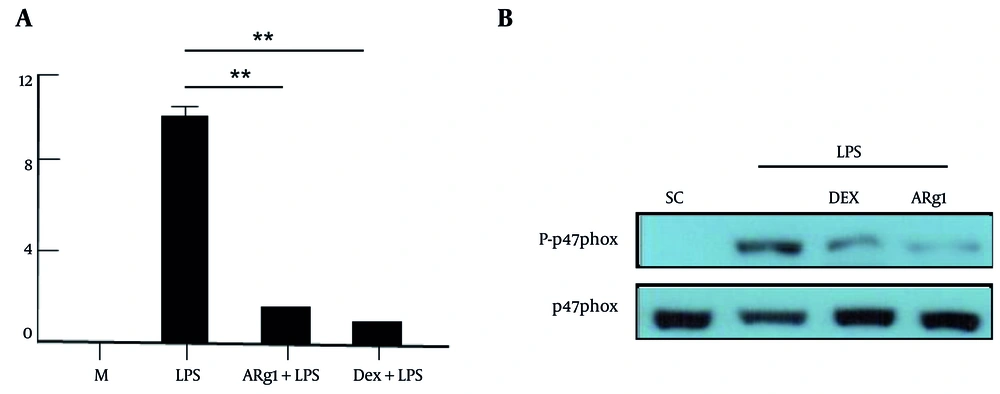
4.5. Compound 3 Inhibits Nicotinamide Adenine Dinucleotide Phosphate Oxidase
The NADPH oxidase was significantly enhanced by LPS in the cells. As shown in Figure 5A, NADPH oxidase also decreased significantly in macrophages treated with ARg1 or Dex compared to the positive control (40 μg/mL, P < 0.01). The phosphorylation process of p47phox stimulates the activity of NADPH oxidase. The results from Figure 5B indicate that the phosphorylation of p47phox induced by LPS was inhibited in cells treated with ARg1 or Dex compared to the positive control (40 μg/mL). These results showed that ARg1 inhibited the phosphorylation of p47phox, leading to the inhibition of NADPH oxidase activity in cells that were incubated with LPS.
Compound 3 (ARg1) has inhibited the activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase induced by lipopolysaccharide (LPS) in cells. The cells were treated with LPS (1 µg/mL) for 30 minutes (A), for 15 minutes (B) after the cells were incubated with ARg1 for 45 minutes. The cells were lysed with a cold lysis solution and each well loaded with 50 μg protein. The phosphorylation of p47phox was analyzed by immunoblotting results (B). Three independent experiments were shown as mean ± standard deviation (SD). M, medium; SC, 10% dimethyl sulfoxide (DMSO); Dex, dexamethasone. ** P < 0.01 is a significant difference.
5. Discussion
Acacia pennata has been used as a popular vegetable in Thailand, Myanmar, and India, where it is viewed as a food rich in minerals, supporting bone health (5). Acacia pennata is also one of the essential ingredients for preparing the famous traditional liquor in the Karbi tribe of Assam, India (5). In Southeast Asia, A. pennata is used to treat diabetes, malaria, and diarrhea (5). The composition of A. pennata includes many types of phytochemicals such as phenols, flavonoids, saponins, and other diterpenoids (13, 14). The role of compounds from A. pennata in anti-inflammatory activity remains unclear. In this study, the extraction and identification of the structure of ARg1 from A. pennata (L.) Willd is published for the first time. The present data suggest an important regulatory role of ARg1 in LPS-stimulated inflammation. Our results showed that the process of several LPS-stimulated proinflammatory cytokines in cells was inhibited by ARg1.
In 2005, extracts from the stems of A. pennata also demonstrated analgesic and anti-inflammatory activity experimentally at the in vivo level (15). In addition, flavonoids isolated from A. pennata inhibited the enzyme COX. Similarly, Rg1 is a triterpenoid saponin found in ginseng (with a structure similar to ARg1) that has anti-inflammatory and antioxidant functions and supports the treatment of nerve injuries. An important activity of ginsenoside Rg1 is considered to be its role as a ligand for the glucocorticoid receptor (16). The LPS has the ability to stimulate the production of pro-inflammatory cytokines and various oxidative reactions (17, 18). In this study, we showed that ARg1 inhibited superoxide production caused by LPS. Furthermore, the activity of NADPH oxidase and phosphorylation of Ser345 of p47phox in LPS-dependent macrophages was also inhibited by ARg1. In addition to its important role in acute and chronic inflammation, ROS can cause several eye-related diseases such as DED, keratoconus, cataracts, and pterygium (3). Therefore, compounds with ROS inhibitory activity during inflammation may improve eye-related diseases. More importantly, this study has shown that the destruction of the ROS generation process in macrophages is partly due to the diminished activity of NADPH oxidase through the inhibition of Ser345 phosphorylation of p47phox by ARg1 when stimulated by LPS. In fact, a component of ginseng with a chemical structure similar to ARg1 is ginsenoside Rg1, which has been shown to have the potential to protect the lens against cataract disease by inhibiting oxidative stress (16). Additionally, the ability to inhibit ROS and the phosphorylation of p38/JNK2 has protected neural stem cells (19).
To date, plant-derived antioxidants are a field of active research in supporting the treatment of cataracts (20). For example, curcumin, flavonoid fractions, and hesperetin have been shown to have protective effects in animal models of cataracts (3). Currently, the role of oxidative stress is important in eye-related diseases. Oxidative stress leads to increased intraocular pressure and is one of the risk factors for glaucoma in older age. The oxidative stress process can also cause mitochondrial dysfunction in glaucoma pathology. In addition, NADPH oxidase (NOX) may also contribute to oxidative stress in glaucoma (3). Many studies have shown that Rg1 has the potential to support the treatment of diseases including inflammation (21) and diseases related to oxidative stress, including eye diseases (5). The data in this study first demonstrate the role of ARg1 from A. pennata, which has a chemical structure similar to ginseng Rg1, in regulating ROS capability by inhibiting p47phox in macrophages. Although antioxidant therapy has been developed for many years, the number of substances that can be clinically tested remains limited. Therefore, the search for plant-derived substances with the ability to inhibit ROS is still very necessary to pave the way for new potential antioxidant drugs. Therefore, the data in this study emphasize the anti-inflammatory ability and ROS inhibition of ARg1 in the treatment of diseases related to inflammation and oxidative stress.
5.1. Conclusions
In this study, ARg1 is a compound that has been extracted and structurally identified from A. pennata. More importantly, ARg1 has the ability to inhibit pro-inflammatory cytokines and ROS. This activity may suggest that ARg1 is a potential factor supporting the effective treatment of inflammatory diseases and certain eye conditions caused by ROS.