1. Background
Morphine is one of the most common drugs for treatment of pain in a clinic, while its successive use leads to development of tolerance and dependence (1, 2). Drug addiction is a chronic and relapsing disease with implication in brain neurobiology and behavior that is associated with drug seeking and persistence of memory (3). Agonist of µ opioid receptors has a key role in induction of analgesic property of opioids. Morphine in high doses causes euphoria, analgesia, respiratory depression, reduction of gastrointestinal motility, miosis, and dependency (4, 5). It has been well confirmed that continuous administration of morphine affects mesolimbic dopaminergic system and leads to development of tolerance and dependence (6, 7). One of the problems after stopping morphine administration is drug withdrawal followed by symptoms such as anxiety, sweating, increased respiratory rate and body temperature, piloerection, restlessness, nausea, diarrhea, lacrimation, abdominal pain, and hyperreflexia (7, 8). In spite of many efforts for exact perception of morphine tolerance and dependence, there are still many questions that are needed to be worked on (1). Based on previous studies, tolerance and dependence include two phases; the induction phase, in which changes occur during administration, and the expression phase, in which changes occur after drug cessation (9). Histamine is one of the first neurotransmitters investigated on morphine tolerance and dependence and studies on it have significantly increased in these years (10). Previous researches demonstrated that subsequent administration of morphine (in tolerance phase) increases histamine turnover in animal brains (11, 12). In addition, investigations on histamine receptors (H1 and H2) showed that histamine H1 receptors play an important role in morphine tolerance and dependence (13-15). Ketotifen is a H1 receptor antagonist and a mast cell stabilizer is used for asthma, atopic dermatitis, seasonal rhinitis, acute and chronic urticaria, allergic conjunctivitis, and food allergy-related (16-18). Nowadays, the use of non-opioid compounds and natural products, especially medicinal plants, has increased in order to avoid unwanted effects of opioids include tolerance, dependence, and euphoria (19). Accordingly, this research was designed to investigate the role of ketotifen, as a non-opioid drug, in morphine tolerance and dependence in mice.
2. Methods
2.1. Animals
The animals (weight 26 ± 3 gr) were housed (8 mice in each cage) with optimum temperature (22 ± 3°C) with an alternating-12hour light-dark cycle with free access to food and water. All mice were used only once, and in order to reduce stress response, the animals were located in the experimental environment 2 days before starting of study. All the ethical issues were considered based on the Ahvaz Medical University Ethical Protocols (AMUEP) and code IR.AJUMS.REC.1394.658 is assigned.
2.2. Drugs
The used drugs including morphine sulfate (Darupakhsh Pharmaceutical Co, Tehran, Iran) was injected subcutaneously (s.c.) and ketotifen fumarate (Hakim Pharmaceutical Co, Tehran, Iran) and naloxone hydrochloride (Tolidaru Pharmaceutical Co, Tehran, Iran) were administered intraperitoneally (i.p.). All drugs were dissolved in normal saline and administered in volume of 10 mL/kg.
2.3. The Mouse Hot-Plate Test
We used Eddy and Leimbach's method (1953) for study of pain reflexes in reaction to heating stimulus in the hot-plate test (20). Each mouse was placed on a hot plate at 55 ± 1°C in a transparent acrylic cage. The response latency was assessed based on either hind licking or jumping reaction, whichever occurred sooner, after first contact with the plate. The cut-off time was set as 20 seconds to prevent damage to the animal. The hot-plate latency was calculated twice before drug injection and twice after; then, the average of the two values was applied to baseline and drug data.
2.4. Evaluation of the Antinociceptive Effects of Ketotifen
Mice were divided into four groups and three doses of ketotifen (2, 4 and 8 mg/kg, i.p.) were assigned to examine. The hot-plate test was carried out on each group at 15, 30, 60, and 90 minutes followed by drug administration.
2.5. The Effect of Ketotifen on Acute Antinociceptive Effects of Morphine
For this purpose, the subeffective dose of ketotifen obtained from the dose-response study was administered 30 minutes before morphine 10 mg/kg and antinociceptive threshold assessed.
2.6. Induction of Morphine Tolerance and Dependence and Assessment of Ketotifen Effects
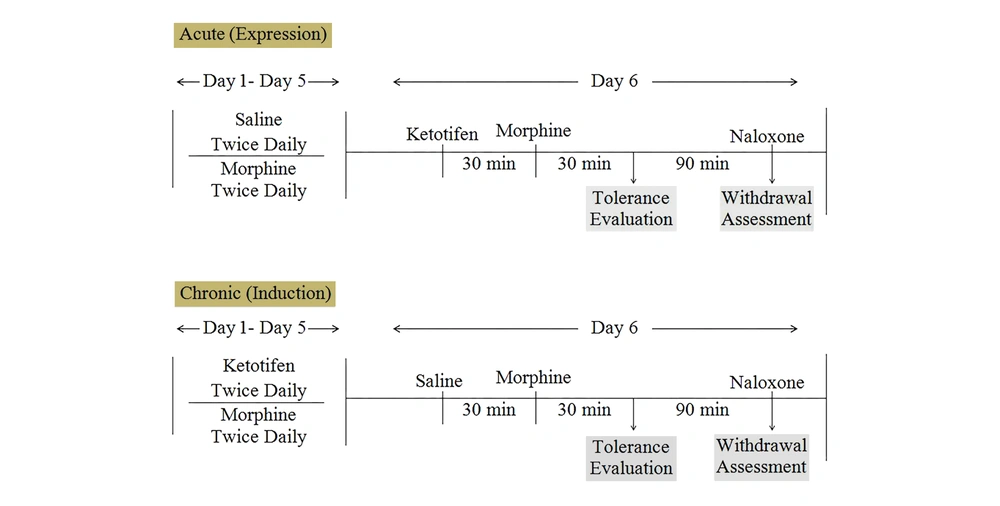
Morphine tolerance and dependence was induced by frequent 11 injection of morphine during six successive days with the doses of 10 mg/kg morphine, twice daily, and the last dose of morphine on the 6th day. Morphine tolerance was assessed in a hot plate model of antinociception. Dependency to morphine was precipitated by naloxone 5 mg/kg after the last dose of morphine in day 6 and withdrawal signs include weight loss and jumping were measured (21). Ketotifen effects were examined in two acute and chronic studies to evaluate the role of ketotifen in the induction and expression of morphine tolerance and dependence (Figure 1).
2.7. The Effects of Acute and Chronic Ketotifen Administration on Morphine Tolerance
On the 6th day of the experiment, the hot-plate test was immediately performed after the last dose of morphine. The degree of tolerance was determined according to the measured latency time reduction in the hot-plate test. Ketotifen, in an acute study was administered in day six of the experiment 30 minutes before morphine and in chronic study before each dose of morphine except on the 6th day.
2.8. The Effects of Acute and Chronic Ketotifen Administration on Morphine Dependence
Naloxone 5 mg/kg, i.p. was injected 2 hours after morphine administration and physical dependence was determined based on jumping and weight loss. For measurement jumping, each mouse was placed on a platform (40 cm length, 25 cm width and 45 cm high) during 30 minutes and the frequency of jumps was recorded. In addition, The Mice's body weight variations were evaluated 1h after the naloxone administration.
2.9. The Statistical Analysis
Values are presented as Mean ± SEM, n = 8 in each group. AUC is the area under the curve relevant to respective graph. One-way ANOVA followed by Tukey's test was used for comparison. Significant difference was assigned at the level P < 0.05.
3. Results
3.1. Ketotifen Antinociceptive Effects
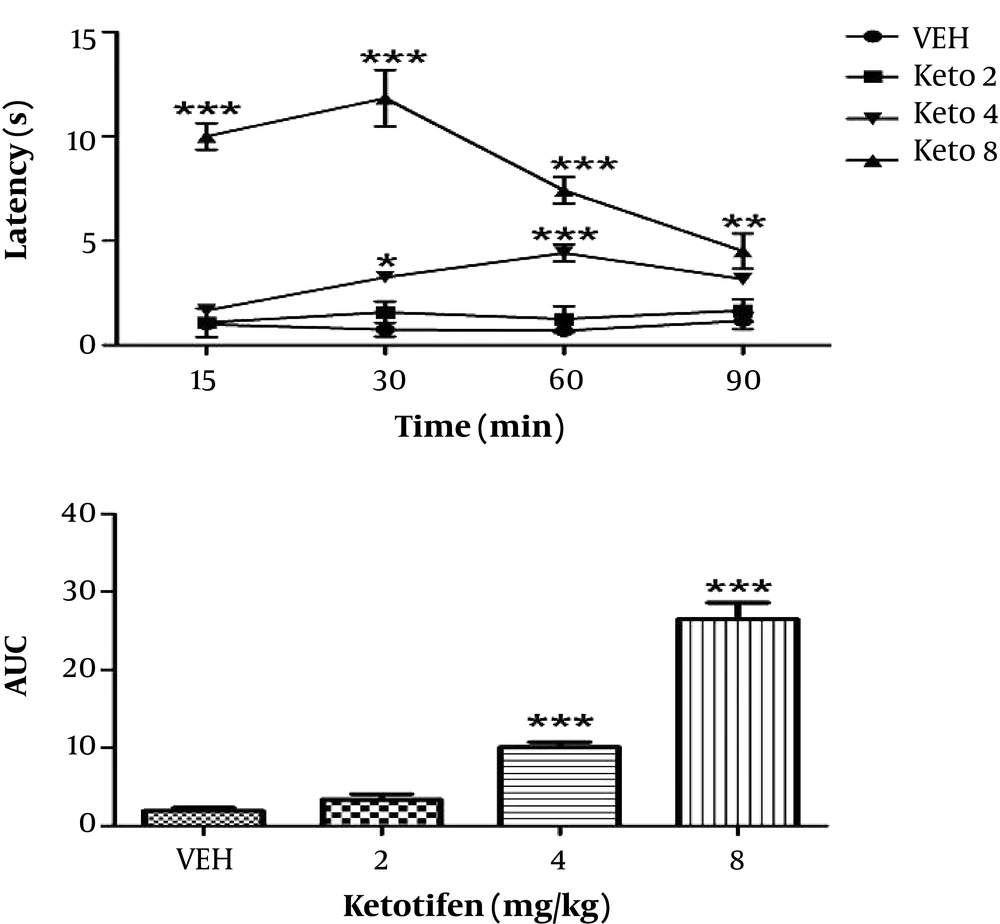
The results showed that increasing doses of ketotifen also increased latency time, so that administration of 4 and 8 mg/kg ketotifen led to significant increase in latency time (P < 0.001), while administration with 2 mg/kg had no significant difference. Moreover, we found out these effects were dose dependent (Figure 2). The dose of 2 mg/kg ketotifen was considered as subeffective dose (the dose that does not change the antinociceptive threshold) and used for subsequent studies.
3.2. The Effect of Ketotifen on Acute Morphine Antinociceptive Effects
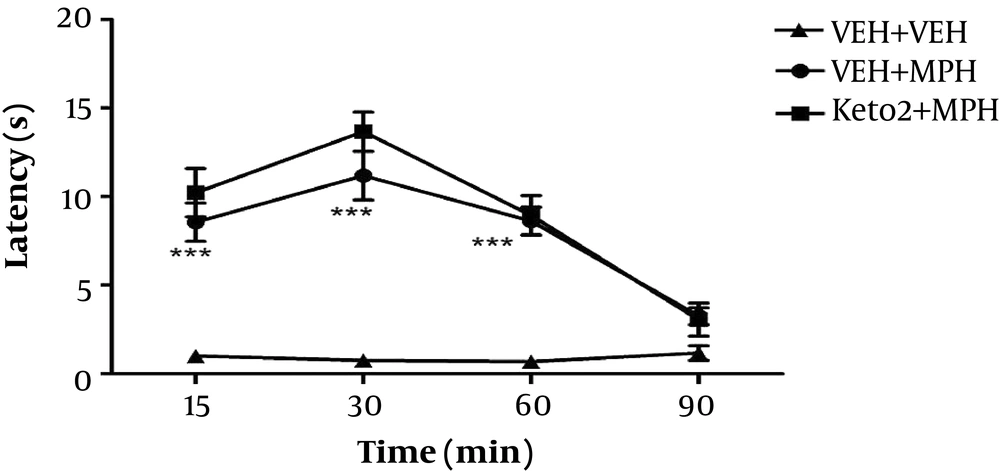
Ketotifen 2 mg/kg did not change latency time of morphine. Effects of morphine and ketotifen co-administered with morphine were significantly different with the vehicle group (P < 0.001) (Figure 3).
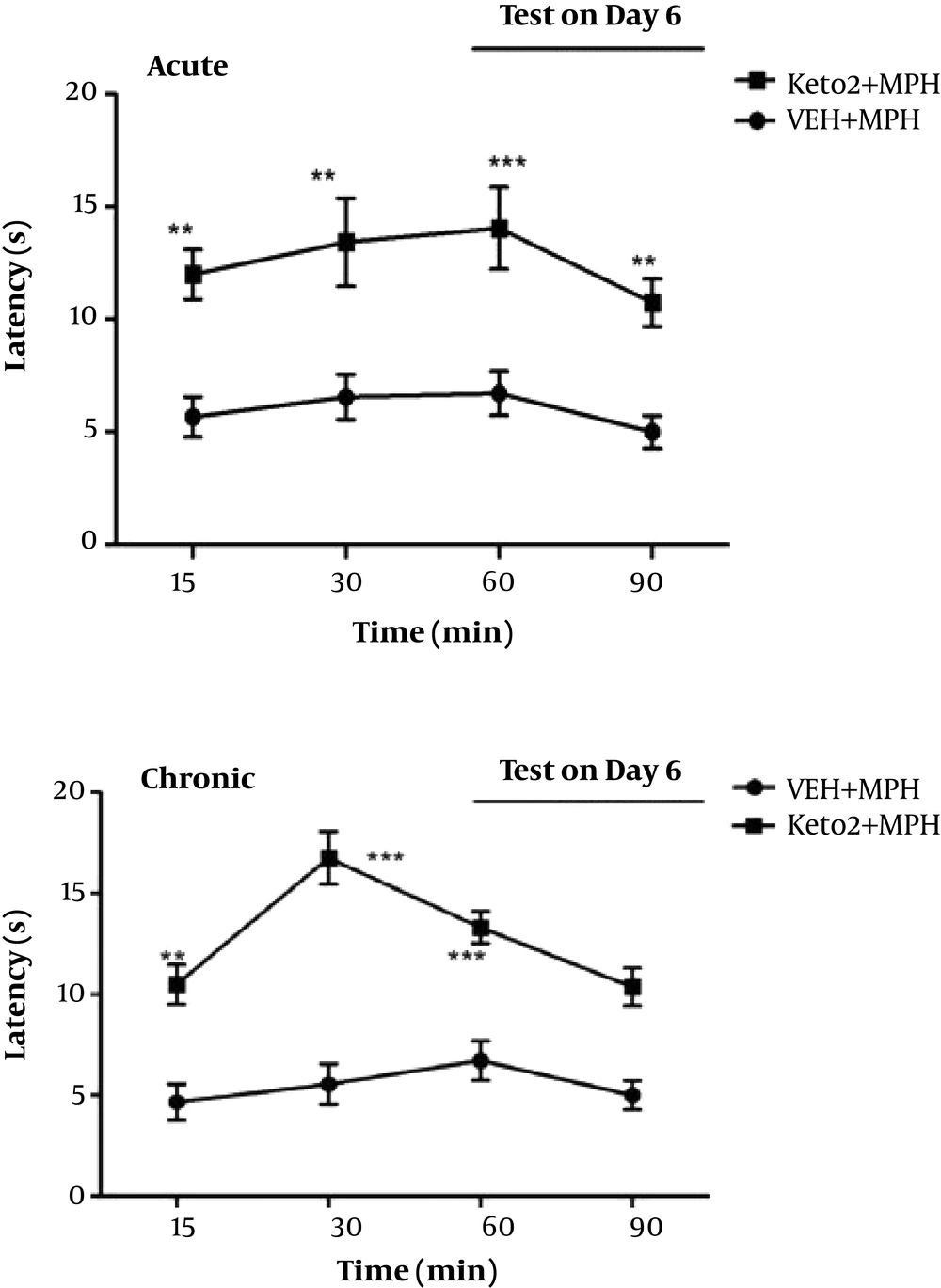
3.3. Acute and Chronic Effects of Ketotifen on Morphine Tolerance
The acute administration of ketotifen resulted in significant prevention of morphine tolerance (Figure 4). For assessment of chronic effect of ketotifen on morphine tolerance, ketotifen administrated 2 mg/kg twice daily prior to each dose of morphine. The results confirmed that ketotifen increase latency time, significantly. This suggests that ketotifen reverses morphine tolerance in mice (Figure 4).
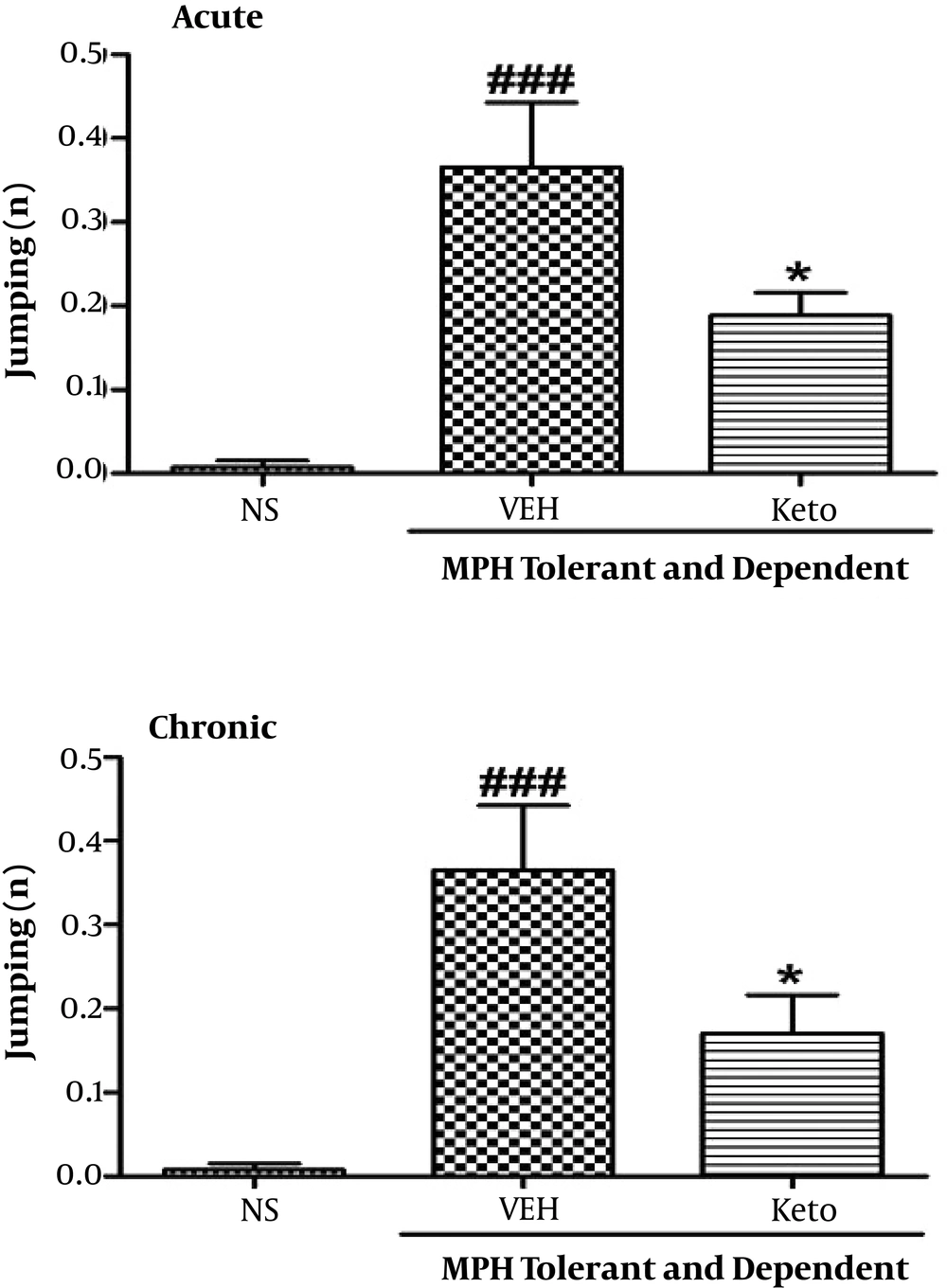
3.4. Acute and Chronic Effects of Ketotifen on Morphine Dependence
As shown in Figure 5, both acute and chronic administration of ketotifen resulted in significant reduction in jumping numbers in comparison to animals treated with morphine (P < 0.05).
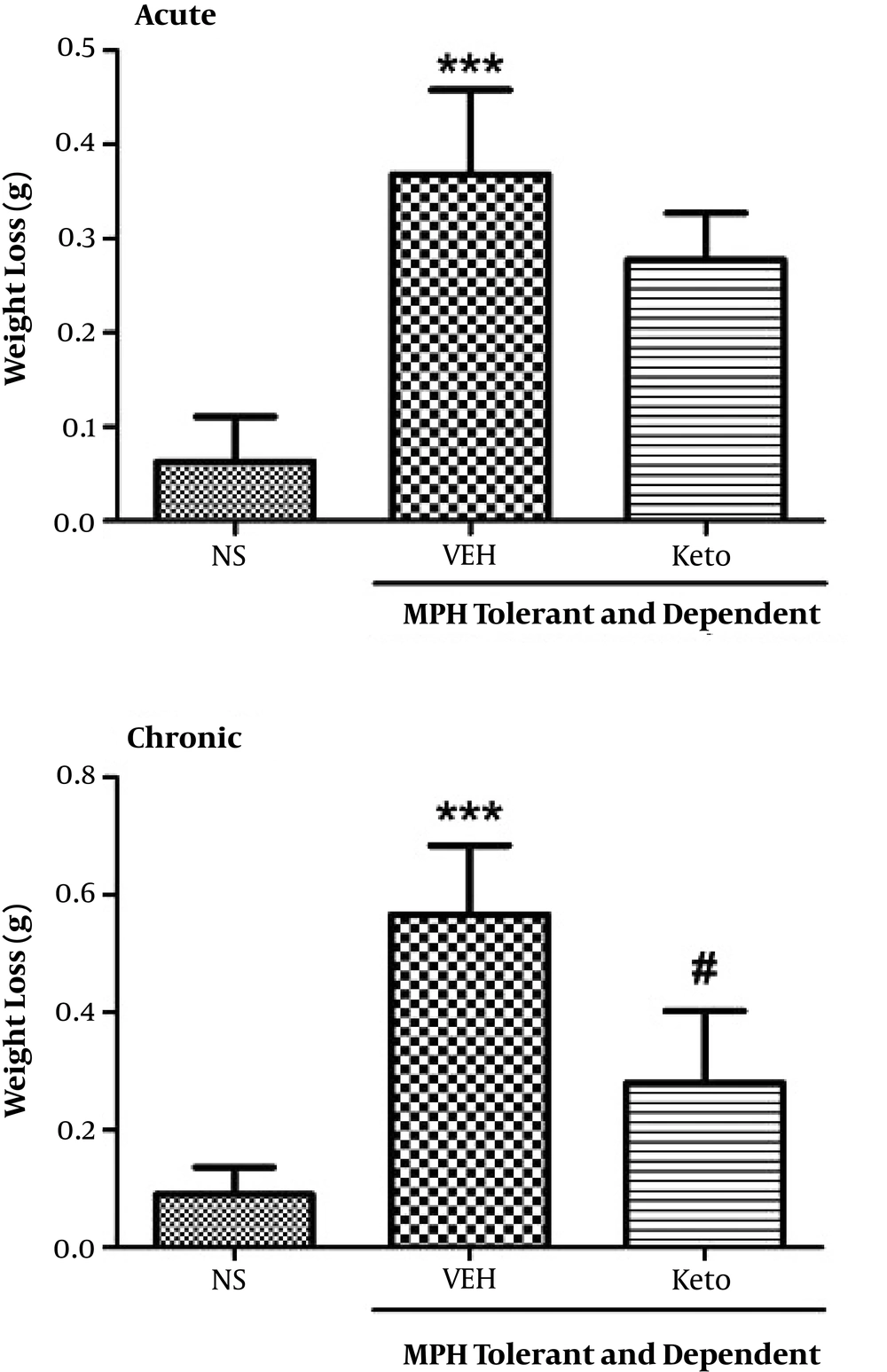
In relation to weight loss determination, we found that chronic administration of ketotifen significantly reduced weight loss (P < 0.05), while weight loss in acute administration of ketotifen was not significant (Figure 6).
4. Discussion
Tolerance is a reduction of pharmacological response to a drug after its long term or repeated use and dose increasing is required to obtain the previous response (22, 23). Dependency can be defined as an altered physiologically state that requires repeated usage or administration for prevention of abstinence and withdrawal syndrome (24). Morphine is used for pain relief in cancer patients and people with chronic pain, however, it has a potential ability to create dependency (4, 25). In this study, we investigated the effect of ketotifen on morphine tolerance and dependence in mice and resulted in novel findings on improvement of morphine tolerance and dependence. Administration of ketotifen showed an antinociceptive effect in a dose-dependent manner and co-administration of ketotifen subeffective dose with morphine (10 mg/kg) did not change morphine latency time. Histamine and its H1 and H2 receptors have a key role in the development of morphine tolerance and dependence (13). It has been reported that H1 receptor antagonists increase the antinociceptive effect of morphine (26). In confirmation, histamine H1 receptor agonist led to increase of morphine tolerance and dependence (14). Histamine H2 receptor antagonist resulted in inhibition of antinociceptive induced by morphine administration (15). Therefore, we find that histamine shares a possible interaction with morphine in opioids tolerance and dependence. The use of opioids increases dopamine release from ventral tegmental area (VTA) to nucleus accumbens (NAc) in mesolimbic, a reward pathway that lead to euphoria and pleasure. The chronic administration of opioids leads to demanding more opioid for releasing the same amount of dopamine from VTA brain cells to NAc through the reward system and ultimately leads to tolerance of opioid. The effect of opioid binding on µ receptors is suppression of noradrenaline release from locus ceruleus (LC) brain cells that causes drowsiness and lowering of blood pressure. However, during the absence of opioids, the high activity of LC leads to manifestation of panic, anxiety, muscle cramps, and diarrhea (27, 28). Moreover, evaluation of dopamine level followed by naloxone-induced withdrawal syndrome showed occurrence of symptoms of withdrawal (for example; jumping) associated with increasing of dopamine level in the brain (29). In addition, it was confirmed that histamine is a stimulatory agent for noradrenergic neurons in the LC (30). It well confirmed N-methyl-d-aspartate (NMDA) receptor antagonist, memantine reduce expression, and induction of morphine-dependence (31). Furthermore, histamine potentiates the NMDA currents in same concentrations that activates H1 and H2 histamine receptors (32, 33). Thus, modulation of morphine actions by NMDA receptors and the regulatory properties of histamine on NMDA receptors can affect initiation and maintenance of tolerance and dependence (34, 35). Moreover, histamine H1 receptor antagonists resulted in dopamine reduction in the neostriatum and NAc (36). Histamine may enhance glutamate signaling through NMDA receptors, which will increase the dopamine extracellular level. These evidences can support effects of ketotifen in improvement of morphine tolerance and dependence. Most of the inputs to the NAc are glutamatergic, and these excitatory inputs can result in releasing of dopamine from striatatum. Histamine H1 receptor antagonists have an inhibitory effect on dopamine activity and monoamine reuptake (36). Morphine causes oxidative stress and triggering formation and release of free radicals through opioids receptors (37) and implications of histamine release well marked by free radicals (38). In this respect, the use of ketotifen can affect the physiopathology of opioids addiction and lead to development of an agent for the treatment of opioids tolerance and dependence. It has been reported that mast cells could be of interest in the resolving of opioids dependence and attenuation of withdrawal signs due to the fact that the number of degranulated mast cells in thalamus significantly increase by naloxone in chronic morphine treated mice (39).
Toll like receptors (TLRs) are important regulators of the innate immune system and activation of mast cells (40). TLRs are involved in neuropathological process of opioids tolerance as well as hyperalgesia and activation of glial by opioids and releasing of pro-inflammatory factors and cytokines (41). Thus, activation of TLRs stimulates the nerves and their inhibition by TLR antagonists reduces neuropathic pain and could relieve us from unwanted effects of opioids including tolerance, dependence, and reward due to opioids (41, 42). This confirms that ketotifen, as an antagonist of TLRs-4 (43), like naloxone can resolve the unwanted effects of opioids. Morphine tolerance implicates the NMDA receptors activation and subsequent formation of nitric oxide by neuronal nitric oxide synthase (35). Histamine activates and increases NMDA currents (32). Overall, histamine is involved in the development of morphine tolerance and dependence, which can be blocked by ketotifen. Finally, we propose that attenuation of tolerance and withdrawal signs can express the modulatory effects of ketotifen and the possible role of histamine in the induction and expression of morphine tolerance and dependence. Thus, ketotifen can be used in conjunction with opioids to prevent tolerance and dependence to opioids and it can also be used in opioids addicted people to prevent withdrawal signs or resolve dependence.