1. Background
Wounds are defined as skin injuries caused by physical or medical conditions (1). Acute wounds, resulting from direct trauma, progress through stages of wound healing over time, while chronic wounds may present challenges, especially in elderly patients and those with multiple morbidities (2). Effective wound management is a dynamic process that requires an understanding of the healing phases and wound classifications (2). Wound healing is a critical physiological process that involves a series of biological events in the body to repair damaged tissues and restore the integrity of the skin or underlying structures. Understanding the physiology of wound healing is essential for developing effective treatment approaches, reducing healing time, and improving outcomes for individuals with wounds. The different stages of wound healing are orchestrated by complex biological mechanisms that aim to restore tissue integrity and function (3, 4).
The process of wound healing involves four complex stages: Hemostasis, inflammation, proliferation, and tissue remodeling, influenced by factors such as age, tissue oxygenation, and underlying medical conditions, and must occur in a specific sequence and time frame to achieve optimal results (5). Both humans and other mammals share common cellular mechanisms for skin repair, such as re-epithelialization and wound closure, which are crucial for the restoration of the epidermis and the prevention of infection (5).
Plants and their extracts have great potential for managing and treating wounds. Herbal remedies for wound healing are not only affordable and cost-effective, but they also appear to be safe, as allergic reactions are rarely caused by these substances (6, 7). Recent developments have highlighted the increasing interest in the use of new plant-based drugs to reduce the use of chemical drugs due to their side effects (8, 9). Quercus brantii, also known as Persian Oak, is one of the medicinal plants commonly used in the southwestern regions of Iran for the treatment of inflammation and stomach wounds. The fruit of the Persian Oak is rich in nutrients and can help the body maintain a healthier and more stable condition (10). The fruit of the Persian Oak contains tannins, gallic acid, and malic acid, which have anti-inflammatory and antioxidant properties. These compounds can help reduce inflammation, relieve pain, and promote wound healing (10).
Nanotechnology has shown great potential in wound healing by providing new materials and strategies for wound dressing and drug delivery. Nanoparticles such as silver, gold, and zinc oxide have been studied for their potential in wound healing (11, 12). Metal nanoparticle-coated wound dressings have demonstrated excellent antibacterial activity and play a vital role in the wound healing process. The unique physicochemical properties of nanomaterials, such as high surface-to-volume ratios, adjustable sizes, shapes, and surface chemistry, significantly enhance their ability to combat bacteria, particularly multidrug-resistant species and biofilms (13).
Metal nanoparticles such as gold, copper, zinc, and iron are increasingly used as carriers for therapeutic agents. They enhance the stability and bioavailability of drugs, allowing for targeted delivery to specific sites within the body. This targeted approach is crucial for minimizing side effects and improving treatment efficacy in conditions such as cancer and inflammation (14). According to studies, gold nanoparticles have therapeutic potential compared to daunorubicin in an animal model of acute myeloid leukemia (15). Additionally, studies show that copper nanoparticles can serve as a promising natural anti-cancer drug for aiding in lung adenocarcinoma treatment (16).
Chemical characterization and analysis of zinc nanoparticles reveal their cytotoxicity, antioxidant, antibacterial, antifungal, and cutaneous wound healing properties (17). Zinc oxide nanoparticles (ZnO NPs) have demonstrated antibacterial, antioxidant, and anti-inflammatory activities, which are crucial for effective wound healing by preventing infections, reducing oxidative stress, and modulating the inflammatory response (18, 19). The ZnO NPs can promote fibroblast proliferation and stimulate cell migration, re-epithelialization, and angiogenesis, which are essential for wound healing (20). These properties of ZnO NPs contribute to their ability to accelerate wound closure and enhance the quality of wound healing (21, 22).
The healing process of wounds is indeed controlled by various growth factors and cytokines. These biological molecules play essential roles in promoting tissue regeneration and improving wound healing (23). Growth factors like epidermal growth factor (EGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), and vascular endothelial growth factor (VEGF) are crucial in the complex process of tissue regeneration and wound healing (23, 24). Cytokines, which are signaling molecules secreted by cells, also contribute significantly to the wound healing process by orchestrating cellular activities that underlie inflammation and healing (25).
The VEGF-A, also known as VEGF, influences vascular permeability, angiogenesis, and the migration of cells like leukocytes and epithelial cells during wound healing (26). It is expressed by several cells, including endothelial cells, fibroblasts, smooth muscle cells, platelets, neutrophils, and macrophages (27, 28). The VEGF level is elevated in several physiological processes, such as estrus, wound repair, and adaptation to hypoxia, or various pathological conditions such as proliferative retinopathies, arthritis, psoriasis, and cancer (29, 30).
The matrix metalloproteinase (MMP) family of proteins is a group of zinc-dependent endopeptidases that play a pivotal role in the degradation and remodeling of the extracellular matrix (ECM). They are involved in various physiological and pathological processes, such as wound healing, tissue remodeling, and cancer progression (31, 32). In wound healing, MMPs are involved in the breakdown and remodeling of the ECM, which is essential for the migration and proliferation of cells during the healing process. They are produced by various cells, including fibroblasts, keratinocytes, and inflammatory cells, and their activity is regulated by multiple factors, such as growth factors, cytokines, and ECM components (31, 32).
2. Objectives
Based on studies regarding the healing effects of Oak fruit on stomach ulcers and the antibacterial and antioxidant effects of ZnO NPs in facilitating the wound-healing process, this study investigated the molecular impact of Persian Oak fruit individually and in combination with ZnO NPs for topical use (ointment) on the healing process of the skin wound excision model.
3. Methods
3.1. Materials
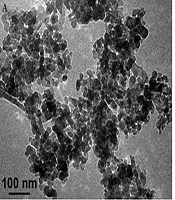
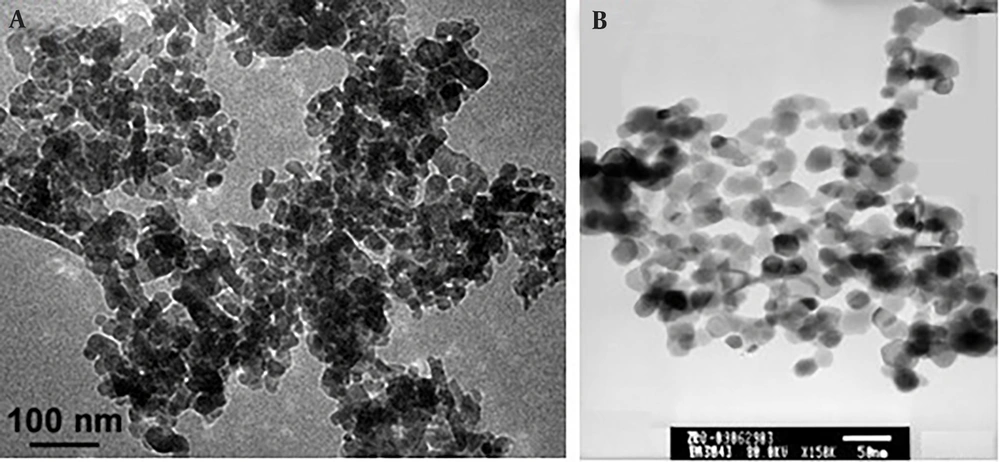
The solvents used were purchased from Merck, and all materials were obtained from Sigma Aldrich chemicals. Zinc oxide (ZnO) nanoparticles (20 nm) were purchased from US Research Nanoparticles, Inc. (Houston, USA). The characteristics of ZnO nanoparticles are shown in Table 1. SEM and TEM images of the nanoparticles provided by the company are shown in Figure 1.
| Name | Purity | APS | Color | Morphology |
|---|---|---|---|---|
| ZnO nanoparticle | 99.8% | 20 nm | White | Nearly spherical single crystal |
Characteristics of Nanoparticles Used in Ointments for Treating Wounds
3.2. Plant Preparation and Extraction
Oak fruit used in this experiment was collected from Lorestan province, Iran. The plant species was identified as Q. brantii by the Department of Horticulture of the Faculty of Agriculture at Shahid Chamran University of Ahvaz, Iran. Collected fruits were washed, shadow dried, and ground in a blender into a fine powder. To prepare oak extracts, a total of 100 g of powdered dried fruit was macerated with 1 liter of 70% ethanol at room temperature for 72 hours. After that, the fruit extract was filtered 2 - 3 times through Whatman No.1 filter paper. The filtrate was then evaporated at 50°C in a rotary evaporator until complete dryness. The dry extract was powdered and stored at 4°C.
3.3. Ointment Processing
The simple ointment base was Vaseline. For plant test ointments, appropriate amounts of Oak plant extract [1.0%, 5.0%, and 20% (w/w)] were added to the simple ointment base. For the nanoparticle test ointment, 0.1% of powdered ZnO nanoparticles were added to the simple ointment base. A combination ointment was also prepared by adding 1.0% Oak extract plus 0.1% ZnO nanoparticles to the simple ointment base. The wound areas were treated with ointments or the reference drug (1.0% phenytoin) topically once a day depending upon group assignment for 14 days.
3.4. Animals and Experimental Groups
This study was authorized by the Medical Ethics Committee of Shahid Chamran University of Ahvaz with the number IR.SCU.REC.1402.062. All animal experiments were conducted following institutional guidelines for animal care and use, which adhered to the international principles of Laboratory Animal Care (NIH publication #85 - 23, revised in 1985). A total of 48 adult male mice (25 - 30 g) were purchased from Jondishapour Ahvaz University of Medical Sciences. The mice were individually housed and fed with an unrestricted diet of food and water under standard conditions (temperature-controlled 20 - 23°C, humidity 45 - 55%, and room on a 12-hour light/dark cycle). After a week, the animals were randomly divided into eight groups of six animals.
3.5. Wound Induction and Treatment
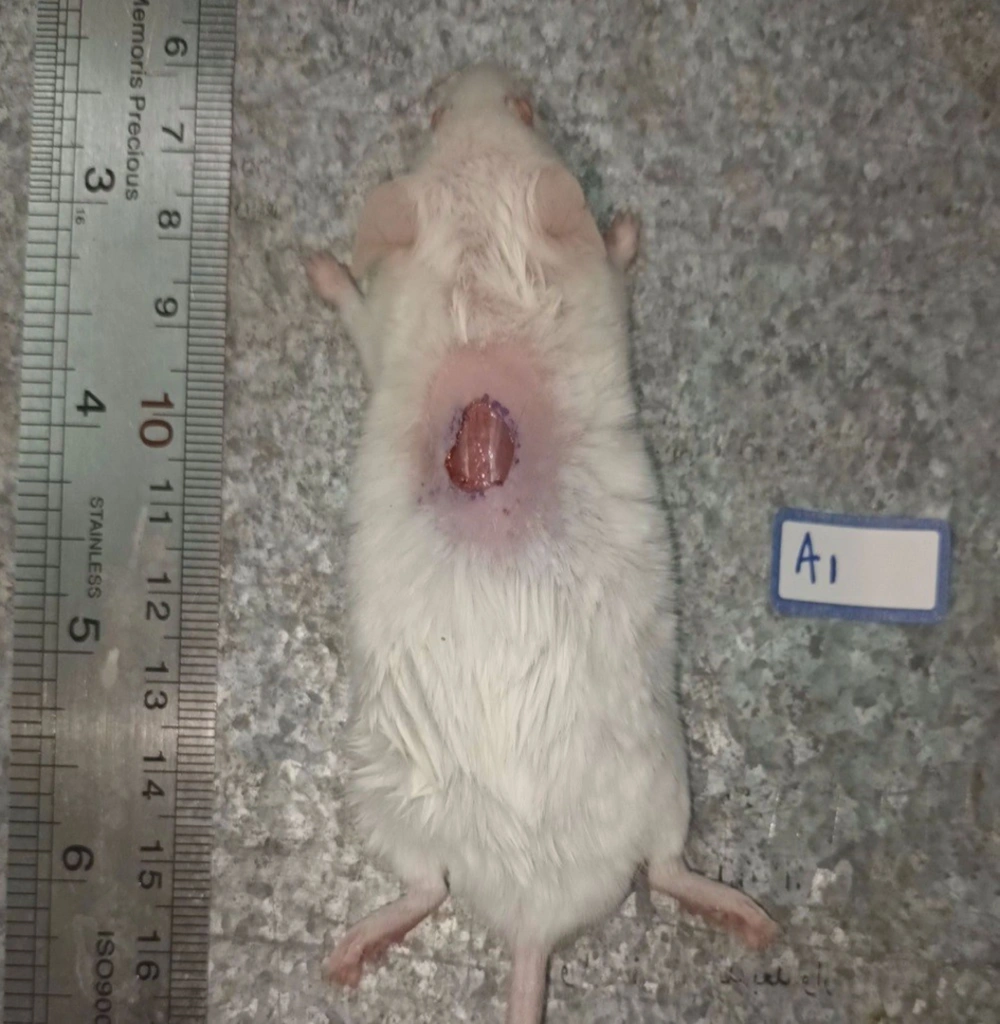
The wound was created at the beginning of the experiment on all testing mice. Each mouse was anesthetized with an intraperitoneal injection of Ketamine (50 mg/kg) and Xylazine (10 mg/kg). The hair on the back of the mice was first shaved, and the wound site was sterilized with 70% ethanol. The skin wound was established by excision of a circular region about 1 cm in diameter on the back of each mouse (Figure 2).
Following the wound induction at time zero, the animals were treated with Vaseline, phenytoin, Vaseline plus Persian Oak extract, and Vaseline plus ZnO nanoparticles as detailed in Table 2. After two weeks, on the 14th day post-wounding, the mice were euthanized using chloroform. The skin tissues from the wound were excised and stored at -70°C.
| Groups | Negative Control | Vehicle Control | Test | Positive Control | ||||
|---|---|---|---|---|---|---|---|---|
| Animal treat | Non-treated | Vaseline | Oak ext. 1.0% | Oak ext. 5.0% | Oak ext. 20% | ZnO NPs 0.1% | Oak ext. 1.0% + ZnO NPs 0.1% | Phenytoin 1.0% |
| Number | 6 mice | 6 mice | 6 mice | 6 mice | 6 mice | 6 mice | 6 mice | 6 mice |
Animals Being Divided into Eight Groups of Six, Receiving Daily Ointment Treatments for Two Weeks
3.6. RNA Extraction and Complementary DNA Synthesis
The total RNA of wound tissues was extracted using the Favorgen Total RNA purification kit (Favorgen-Taiwan). The concentration of RNA samples was measured by spectrophotometer (Nanodrop 2000-Germany). First-strand complementary DNA (cDNA) was prepared by reverse transcription using the PrimeScript™ RT Reagent kit (Takara Bio Inc., Japan) according to the manufacturer's instructions.
3.7. Real-time Polymerase Chain Reaction
The cDNA was used for real‑time polymerase chain reaction (PCR) using the SYBR Premix Ex Taq II (Tli RNaseH Plus) kit (Takara Bio Inc., Japan) on the Lava96 Real-time PCR Detection System (DaanGene Co. Ltd). Real‑time PCR was executed for 50 cycles at 94°C for 15 seconds, 60°C for 15 seconds, and 72°C for 30 seconds, using the primers for normalizing GAPDH as a standard housekeeping gene against target genes (VEGF, MMP2). Primers were designed with Primer 3 according to the cDNA sequences of VEGF, MMP2, and GAPDH in Gene Bank as shown in Table 3.
| Gene | Gene Bank Accession Number | Forward Primer (5' > 3') | Reverse Primer (5' > 3') |
|---|---|---|---|
| GAPDH | NM_001289726.1 | ATGACTCTACCCACGGCAAG | CTGGAAGATGGTGATGGGTT |
| VEGF-mice | NM_001025250 | CTGCTGTAACGATGAAGCCCTG | GCTGTAGGAAGCTCATCTCTCC |
| MMP2-mice | NM_008610 | CAAGGATGGACTCCTGGCACAT | TACTCGCCATCAGCGTTCCCAT |
The Sequence of Designed Primers for Each Gene Shown as Forward and Reverse
Real‑time PCR was performed in duplicate for every cDNA. Expression in wound tissues treated with plant extract, ZnO nanoparticles, and both was compared with the control (Vaseline-treated tissues) after normalization with GAPDH. To determine the difference in gene expression between groups, the data were analyzed using GraphPad Prism (version 8). It calculates the relative expression of group means for target genes versus the normalizing housekeeping gene.
3.8. Statistical Analysis
All real-time experiments were repeated twice. Data were represented as mean value ± standard deviation. Statistical analysis was performed with GraphPad Prism (version 8) software. One-way analysis of variance (ANOVA) was employed and followed by Dunnett's multiple comparisons test to compare means in different groups with each other. For all statistical tests, the level of statistical significance was set at P < 0.05. To determine the difference in gene expression, the data were analyzed using the ΔCt values for each gene.
4. Results
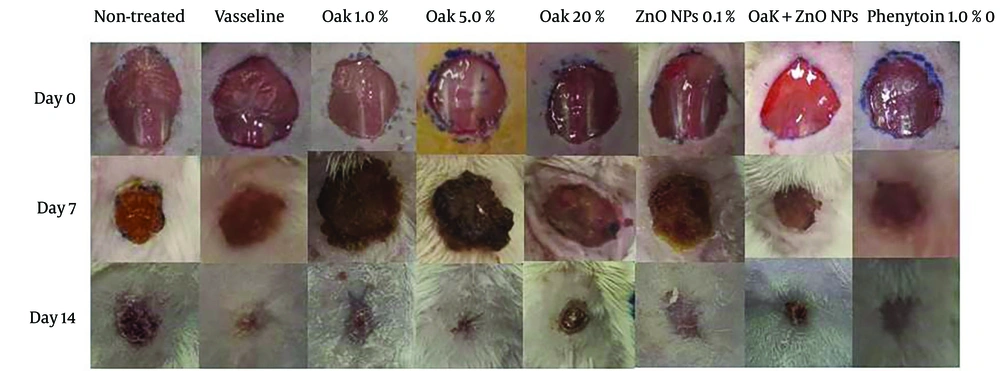
An excision wound model was used and treated with Oak extract and ZnO NPs in eight experimental groups to evaluate the wound healing process over 14 days. Wound healing was analyzed daily, and selected wound images on days zero, 7, and 14 are shown in Figure 3. The results showed that wounds treated with Oak extract 20%, ZnO NPs 0.1%, and Oak 1% plus ZnO NPs 0.1% healed at a faster rate on days 7 and 14, indicating these components accelerated wound healing in these groups (Figure 3).
4.1. RNA Evaluation
Total RNA was extracted from the wound skin tissues. The Nanodrop results showed that the mean concentration of samples was 200 ng/µL. Only RNA samples exhibiting an A260/A280 ratio of more than 1.8 were used for cDNA synthesis.
4.2. Gene Expression Analysis
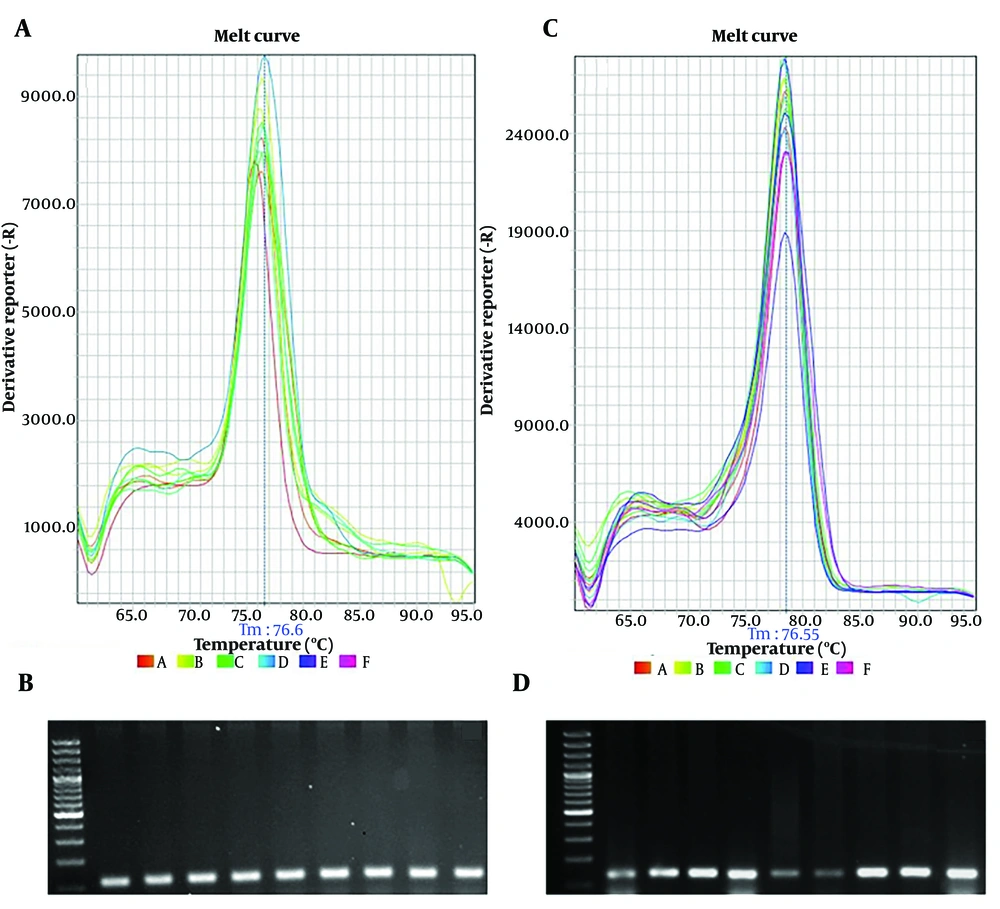
The present study determined changes in the expression of VEGF and MMP2 genes by real‑time PCR in wound skin tissue treated with Persian Oak extract and ZnO nanoparticles. Before data analysis, melting curves were obtained for each gene. The curves confirmed the accuracy of the peak corresponding to the gene of interest and the absence of primer dimers. Agarose gel electrophoresis of real-time products also showed the accuracy of products (Figure 4A - D).
A, the melt curve of vascular endothelial growth factor (VEGF) primers confirmed the peak corresponding to the VEGF gene. B, agarose gel electrophoresis showed the 119 bp length of VEGF real-time products. C, the melt curve of matrix metalloproteinase 2 (MMP2) primers confirmed the peak corresponding to the MMP2 gene. D, agarose gel electrophoresis showed the 139 bp length of MMP2 real-time products.
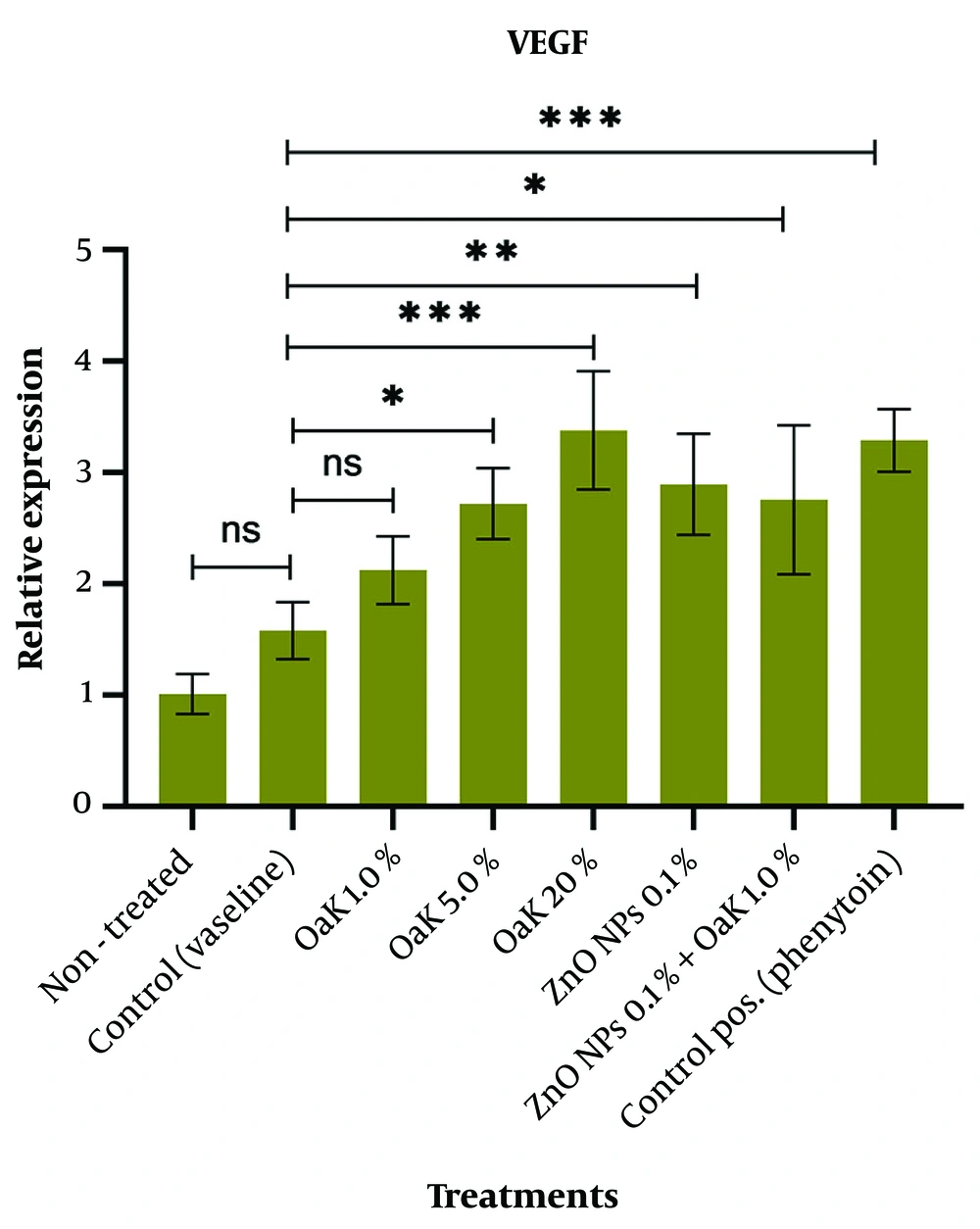
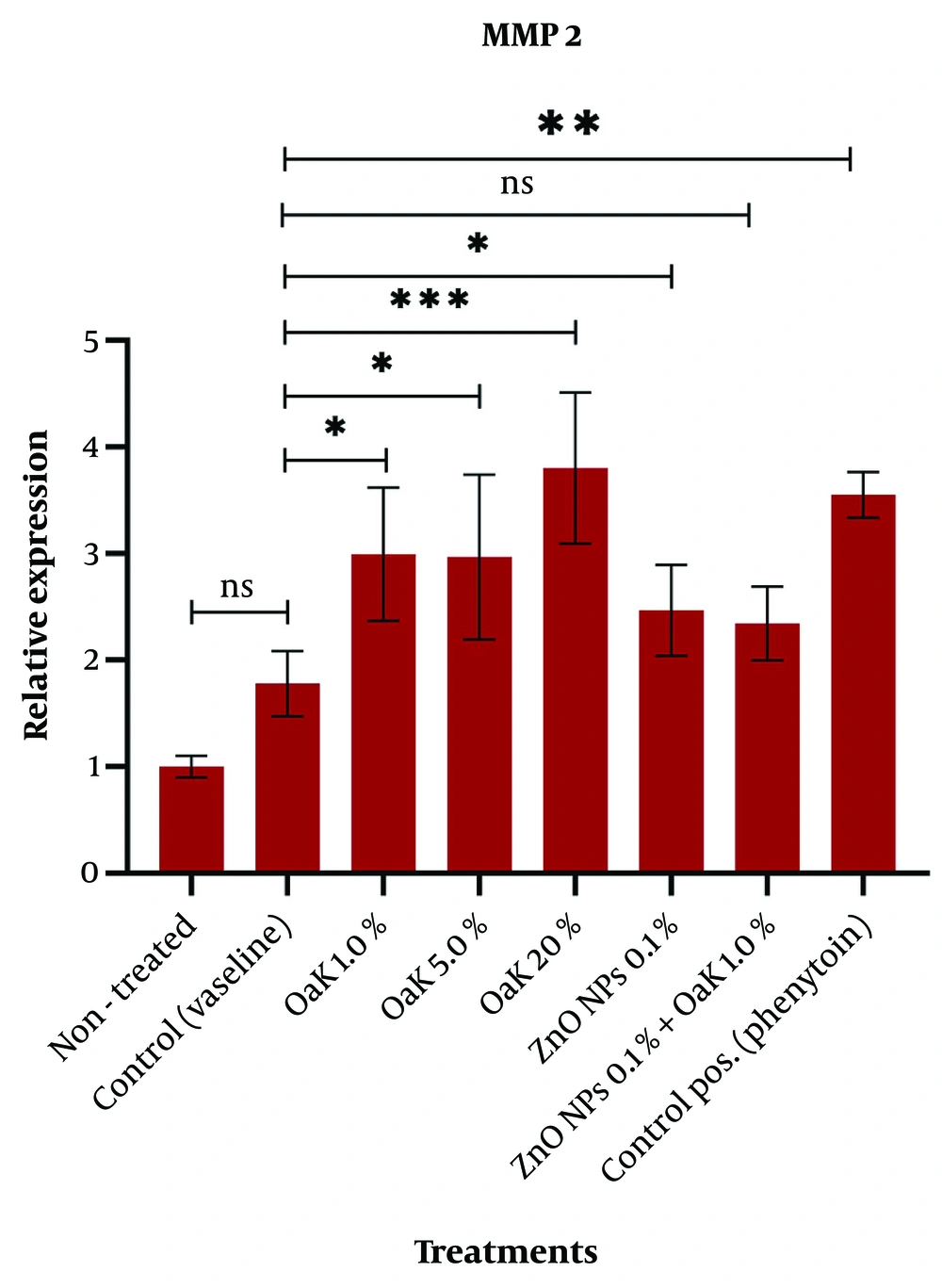
We evaluated the relative expression of VEGF and MMP2 genes in the skin tissues treated with Persian Oak extract (1.0%, 5.0%, and 20%), ZnO NPs (0.1%), and Oak extract (1.0%) plus ZnO NPs (0.1%). Real-time analysis showed that the maximum fold change of both genes was in the Oak (20%) group (Table 4). The fold change indicates the relative expression of the target gene compared to the internal control gene.
| Gene Groups (N = 6) | VEGF | MMP2 |
|---|---|---|
| Non-treated | 1.01 ± 0.18 | 1.00 ± 0.10 |
| Vaseline | 1.57 ± 0.25 | 1.78 ± 0.30 |
| Oak 1.0% | 2.12 ± 0.30 | 2.99 ± 0.62 |
| Oak 5.0% | 2.71 ± 0.31 | 2.96 ± 0.77 |
| Oak 20% | 3.37 ± 0.53 | 3.80 ± 0.70 |
| ZnO NPs 0.1% | 2.89 ± 0.45 | 2.62 ± 0.25 |
| ZnO NPs 0.1% + Oak 1.0% | 2.75 ± 0.66 | 2.34 ± 0.34 |
| Phenytoin 1.0% | 3.28 ± 0.28 | 3.55 ± 0.21 |
The Fold Change Mean and Standard Deviation of Vascular Endothelial Growth Factor and Matrix Metalloproteinase 2 Genes in the Studied Groups
Our statistical findings showed that the relative expression of the VEGF gene in the skin tissues treated with Persian Oak extract (5.0% and 20%) increased significantly compared to non-treated and Vaseline control tissues (P = 0.017 and P = 0.0003, respectively). In contrast, the relative expression of this gene in the skin tissues treated with 1% Oak extract did not show any significant change (P = 0.442) (Figure 5). Additionally, our results revealed that the expression of the VEGF gene in the wound skins treated with 0.1% ZnO nanoparticles and 0.1% ZnO nanoparticles plus 1.0% Oak extract increased significantly compared with the wound skins of controls (P = 0.006 and P = 0.014, respectively) (Figure 5). The drug phenytoin (1.0%) had the most pronounced effect on the expression of the VEGF gene (Figure 5).
The relative expression of the MMP2 gene in the skin tissues treated with Persian Oak extract (1.0%, 5.0%, and 20%) increased significantly in comparison to non-treated and Vaseline control groups (P = 0.041, P = 0.047, and P = 0.0007, respectively) (Figure 6). The expression of the MMP2 gene in the wound skins treated with 0.1% ZnO nanoparticles increased significantly compared with the control wound skins (P = 0.045), while the expression of the MMP2 gene in the wound skins treated with 0.1% ZnO nanoparticles plus 1.0% Oak extract did not change significantly (P = 0.563) (Figure 6).
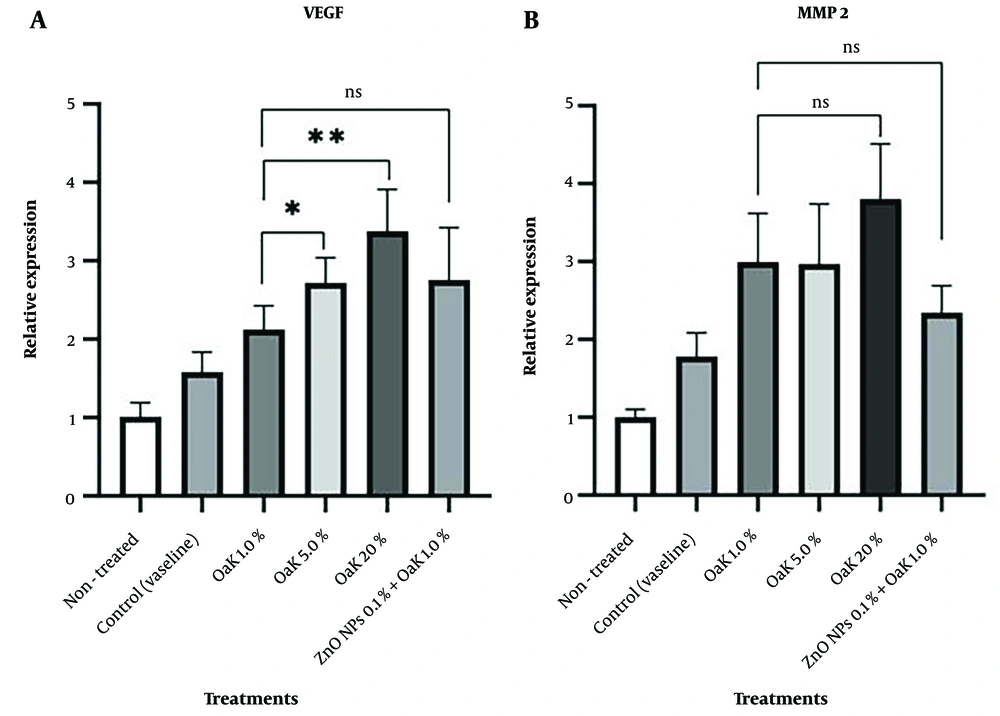
Increasing the concentration of Oak extract upregulated the expression of the VEGF gene significantly (P = 0.043) (Figure 7A), but no significant change was seen in MMP2 gene expression with increased concentration of Oak extract (P = 0.072) (Figure 7B). The expression of both target genes (VEGF and MMP2) in the wound tissues treated with Oak extract plus ZnO nanoparticles did not change significantly in comparison to the wound tissues treated with Oak extract or ZnO nanoparticles separately (P = 0.121 and P = 0.281, respectively) (Figure 7A and B).
5. Discussion
Medicinal plants and their bioactive components have been widely used and studied for their wound-healing properties. Recent studies have highlighted the potential of herbal drugs in enhancing wound healing efficiency (7). Plant-derived biomaterials have gained significant attention in various fields over synthetic materials, including cost-effectiveness, biocompatibility, and environmental sustainability (33, 34). However, it is important to note that the safety and effectiveness of plant materials in wound healing depend on several factors, including their design, interactions with biological systems, and the results of rigorous preclinical and clinical trials (35).
In the present study, the wound-healing activity of Q. brantii extract was investigated by examining whether this extract affects the expression level of VEGF and MMP2 transcripts in the wound tissues. Our results indicated that Q. brantii extract significantly over-expressed the VEGF and MMP2 genes. The VEGF is a polypeptide protein that mediates angiogenesis, vascular permeability, and inflammation by binding to receptors. The VEGF is mainly expressed in endothelial cells of blood vessels and lymphatic vessels, fibroblasts, smooth muscle cells, platelets, neutrophils, and macrophages (36). Studies have shown that VEGF transcription is elevated in acute wounds, contributing to the healing process by stimulating angiogenesis, collagen deposition, and epithelialization (37, 38).
Shams et al. used overexpressing VEGF in fibroblasts to evaluate the effects of VEGF regulation on wound healing. They indicated that the VEGF overexpression in fibroblast cells exhibited a remarkable difference in the expression of migration markers compared to the control group. Fibroblasts accelerate and improve wound repair by secreting ECM and other growth factors (38).
The MMP2 plays a significant role in wound healing by facilitating the migration of fibroblasts during angiogenesis and ECM remodeling (39). Different studies revealed that MMPs that originated or developed from epithelial cells accelerate the inflammatory stage of wound healing. Parks et al. demonstrated that MMPs are prominently expressed in the inflammatory cells. MMPs regulate the transepithelial migration of leukocytes and the partition of signaling proteins such as chemokines in the inflammatory phase (40). Several studies have demonstrated that following skin wounding, the expression of MMPs was increased (41-44). Wu et al. found that the overexpression of the proto-oncogene Src accelerated keratinocyte migration in vitro and promoted wound healing through the induction of MMP2 protein expression (45).
Various plant products have been used to treat wounds over the years. Phytoconstituents derived from plants need to be identified and screened for antimicrobial activity to manage wounds (46). It was found that the hydro-alcoholic extract of Q. brantii contains active components such as tannins and flavonoids (47). Additionally, it has been reported that Q. brantii has antioxidant and antimicrobial properties (48). Similar results have been reported by Karimi and Moradi for Q. brantii, possibly due to its antioxidant potential (49). Mohammadi et al. demonstrated that the topical extract of Teocurium polium or Q. brantii can be used to treat burn injuries (50). The search results provide evidence that Q. brantii ointment can significantly improve wound healing in animal models, particularly in terms of wound contraction, epithelialization period, hydroxyproline content, and tensile strength (51).
Our findings provide strong evidence supporting the efficacy of Q. brantii in promoting wound healing. This suggests that Q. brantii could be a valuable natural treatment option for enhancing wound healing outcomes. Further research is warranted to explore the underlying mechanisms and to evaluate the potential for clinical applications in human patients.
Many studies have shown the efficacy of zinc in wound healing. Zinc-containing preparations improve wound healing by controlling bacterial infection in the wound tissue (52, 53). The ZnO NPs have been shown to possess significant antibacterial properties, which can be particularly effective in preventing wound infections by inhibiting bacterial colonies (54). We evaluated the effect of ZnO NPs (0.1%) on the expression of VEGF and MMP2 genes in the wound tissues. Our findings showed a significant increase in the expression levels of both target genes due to the presence of ZnO NPs. These results are consistent with previous studies. Wang et al. demonstrated that the application of ZnO NPs as an antimicrobial agent significantly improved the wound closure rate in rats compared to the untreated control group (55).
The ZnO NPs are increasingly recognized for their effectiveness in treating a variety of bacterial and fungal skin infections due to their potent antimicrobial properties. Dhanasegaran et al. studied the antimicrobial effect of ZnO NPs on Pseudomonas aeruginosa and demonstrated a significant inhibition of the growth of P. aeruginosa treated with ZnO NPs (56). Tayel et al. compared the antibacterial properties of zinc oxide powder and ZnO NPs. They found that ZnO NPs are a more effective antibacterial agent than traditional zinc oxide powder in controlling bacterial growth (57).
5.1. Conclusions
The current study revealed that Oak extract and ZnO NPs are both effective for wound healing by increasing the expression of VEGF and MMP2 genes at the wound site. However, their combination did not exert an intensifying effect on the expression of the genes and wound healing. It seems that part of the wound healing effects of Oak extract and ZnO NPs overlap and occur through increasing the expression of VEGF and MMP2 genes. To further investigate the effectiveness of the combination of Oak extract and ZnO nanoparticles (NPs) in wound healing, several additional factors should be examined. It is suggested that this combined topical medicine be tested in other concentrations of Oak extract and ZnO nanoparticles and should be further examined on other factors involved in wound healing. However, preclinical studies should provide important information about the safety and efficacy of plant materials, their toxicity, and the long-term stability of nanomaterials for medicinal purposes.