1. Background
One of the important areas of research in nanotechnology is investigating the production of nanoparticles (NPs) of different sizes, shapes and controlled dispersion (1). Currently, nanometal particles have gained significant attention (particularly silver), due to their broad uses in the areas of electronics, material science and medicine (2). Unfortunately, most of the synthetic methods are expensive, toxic and not ecofriendly (3), and thus there is an ever growing need for green chemistry to use biosystems for production of nanoparticles (4). Application of plant extracts is the preferred method over other biological processes, because the complex process of maintaining cell cultures are removed in this technique and it is also suitable for large-scale synthesis of nanoparticles (5). So far many reports have been published in literature on the biogenesis of silver NPs using several plant extracts (6-32).
2. Objectives
In this study, green synthesis of Ag NPs using Juglans regia leaf without the presence of hazardous and toxic solvents and wastes were investigated.
3. Material and Methods
Juglans regia L. and Camellia sinensis L. (a previously proven plant as positive control) leaves were freshly obtained from Isfahan Agricultural and Natural Resources Research Center. Extractions were done following washing, air drying at room temperature and powdering the plant. Ethanolic extract was prepared by percolation (48 h), yielding 100 g plant powder extracted with hydro alcoholic solution (70%, 500 mL) using a 2 L percolator. The extract was concentrated to 50 mL. To prepare boiling water extract, 20 g plant leaf powder was boiled with 100 mL HPLC grade water (15 minutes). After filtering (Watman No: 4), clear extract was obtained (21).
The reaction mixtures contained (final concentration): AgNO3 (1 mM) as the substrate, glucose (560 Mm) as the electron donor, phosphate buffer (pH =7, 25 mM), plant extract or powder as the biocatalyst and ethanol 70% as the solvent in reaction mixture. The aforementioned ingredients were added in appropriate volumes into Duran® bottles (100 mL) and were incubated and shaken (70 rpm) at room temperature. Samples (1.5 mL) were taken from the reaction mixtures at different times, and the absorbance (450 nm) of the colloidal suspensions of silver nanoparticles (hydrosols) was read freshly (without freezing) and immediately after dilution (1:80). The absorption spectra were measured on a Shimadzu (UV mini-1240) spectrophotometer. The UV/Vis absorption spectrum of colloidal Ag was examined to monitor the bioproduction of silver nanoparticles. The ultraviolet visible absorption spectra of samples were evaluated at different time intervals after the start of the reaction. The λmax was about 450 nm in all samples. Transmission electron microscopy (TEM) was performed on selected samples in order to investigate the process of formation of silver nanoparticles and to study their size and shape. Samples for TEM were prepared by drop-coating the Ag nanoparticle solutions on to carbon-coated copper grids. Micrographs were obtained using an EM 900 ZEISS transmission electron microscope.
4. Results
4.1. Visual Observation
When plant extract or powder was exposed to Ag+ ions (AgNO3, 1 mM), the color of the reaction mixture turned yellowish brown, and then dark brown, which was consistent with the former studies, and was considered as the production of colloidal suspension (hydrosol) of silver nanoparticles (10, 21, 30). The appearance of dark brown is due to excitation of surface plasmon resonance in the nanoparticles.
4.2. Monitoring Synthesis of Silver Nanoparticles
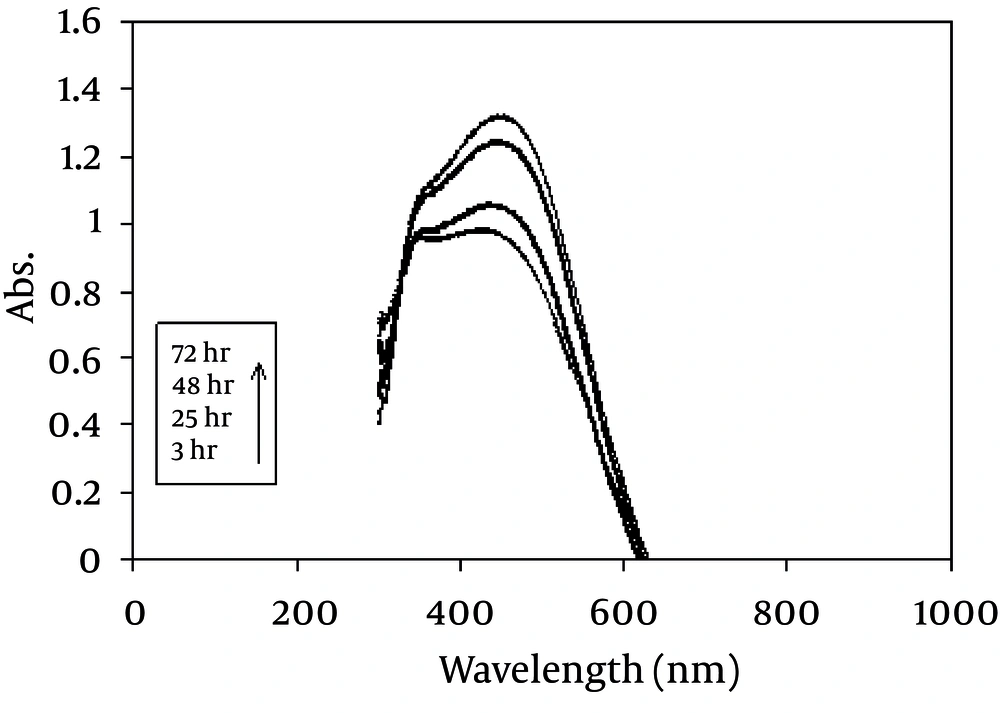
During the reaction period (from 3 h to 72 h), an increase in absorbance was observed in this wavelength, which is likely due to the increase in the production of colloidal silver nanoparticles ( Figure 1 ). Thus, the absorption spectrums of Ag hydrosols obtained in previous studies were confirmed (10, 26).
4.3. Observations Regarding Preparation of Samples for Analysis
In the samples, separation of biomass eliminated the interference in reading UV/V is absorbance (450 nm) of colloidal silver nanoparticles. It was assumed that sample filtration with filter discs (0.2 µm) or by centrifugation (15294 g, Eppendorf centrifuge 5417 R) could be useful. However after using both of these methods, the absorbance (450 nm) of colloidal suspension of nanoparticles decreased greatly to near zero and the color (yellow to brown) disappeared. This might be due to the aggregation of nanoparticles or absorption of them in the biomass. Therefore, filtering and centrifuging the samples revealed negative effects on nanoparticles and should be avoided. Instead of these two methods, samples were diluted (dilution factor = 80) and their absorbance was recorded at 450 nm.
4.4. Nanosilver Particle Formation by Ethanolic Extract
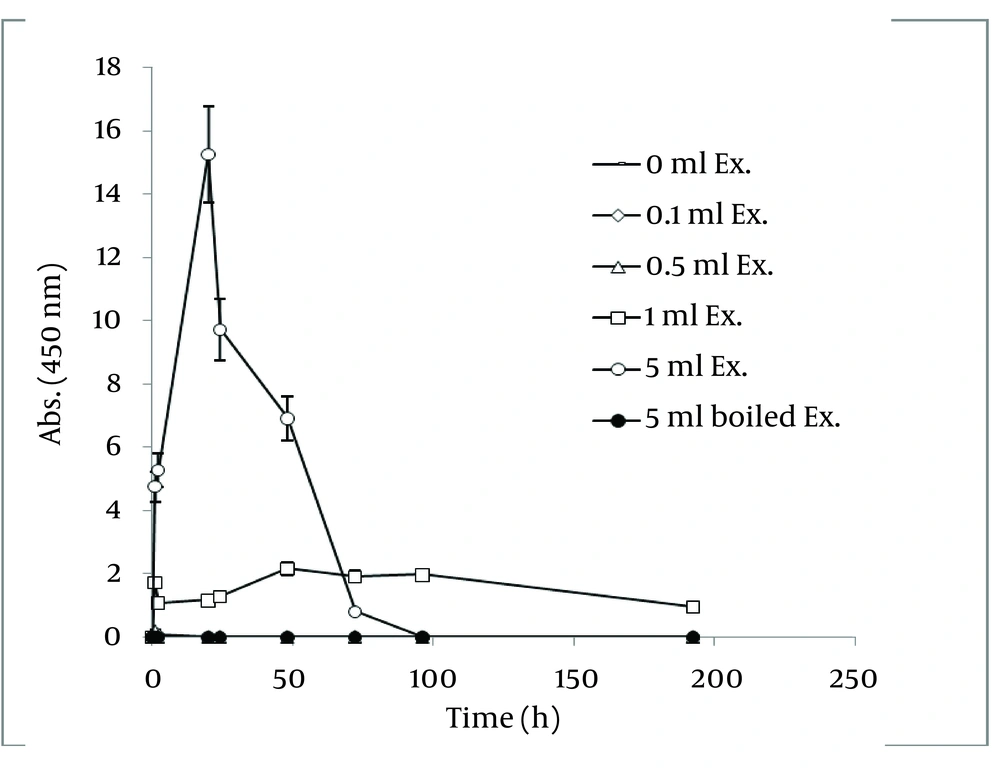
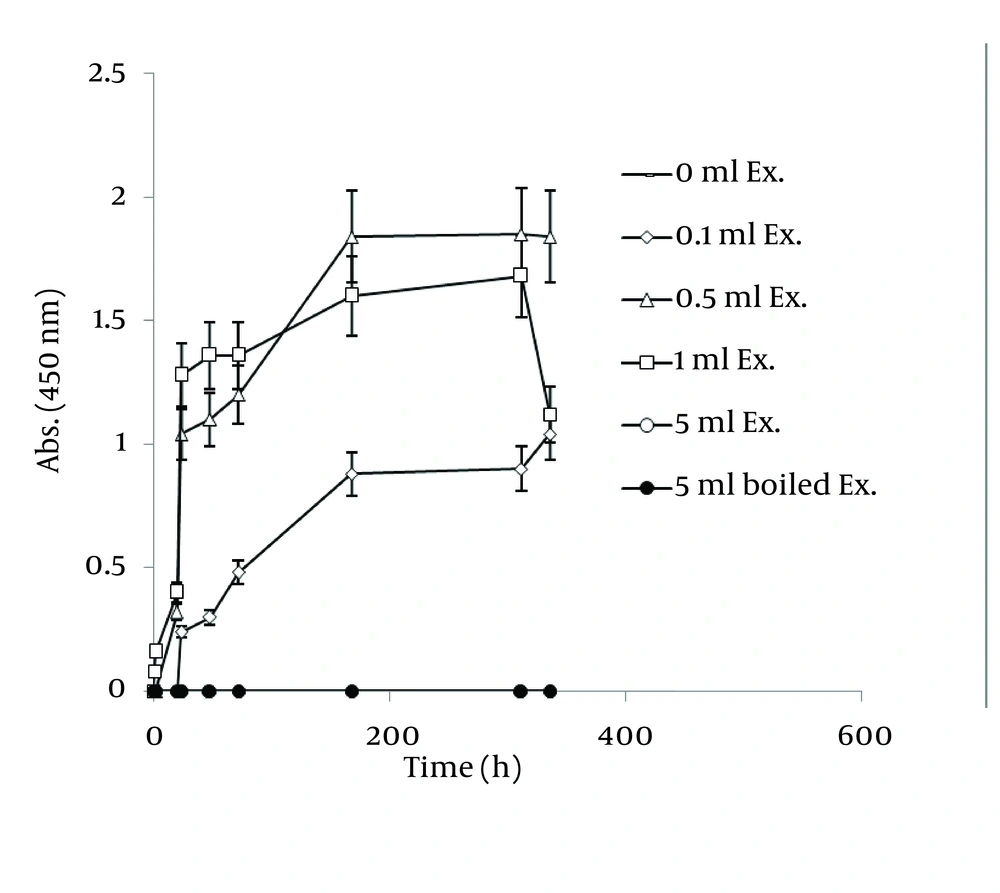
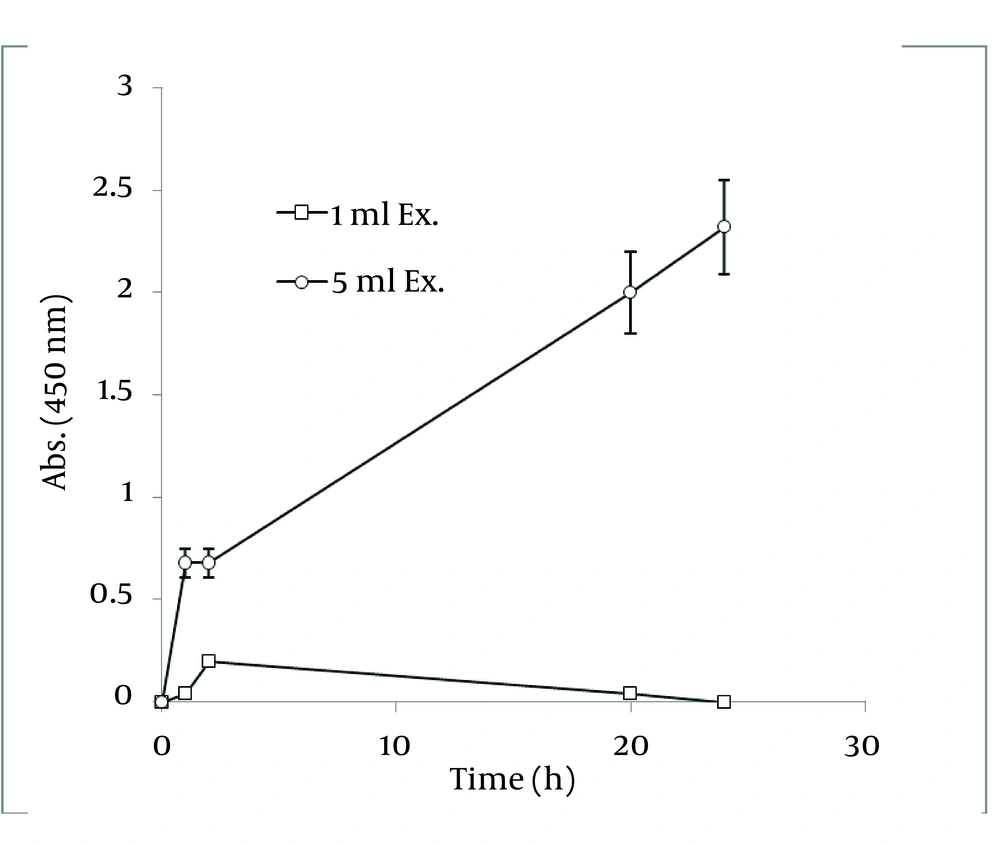
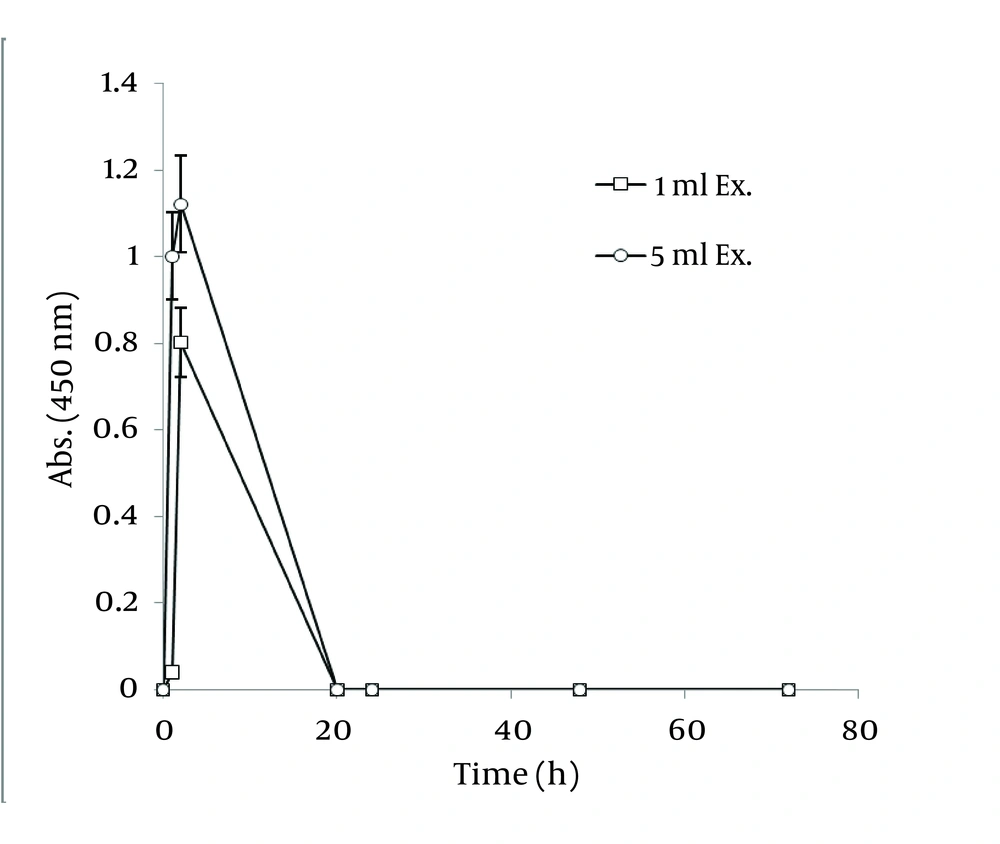
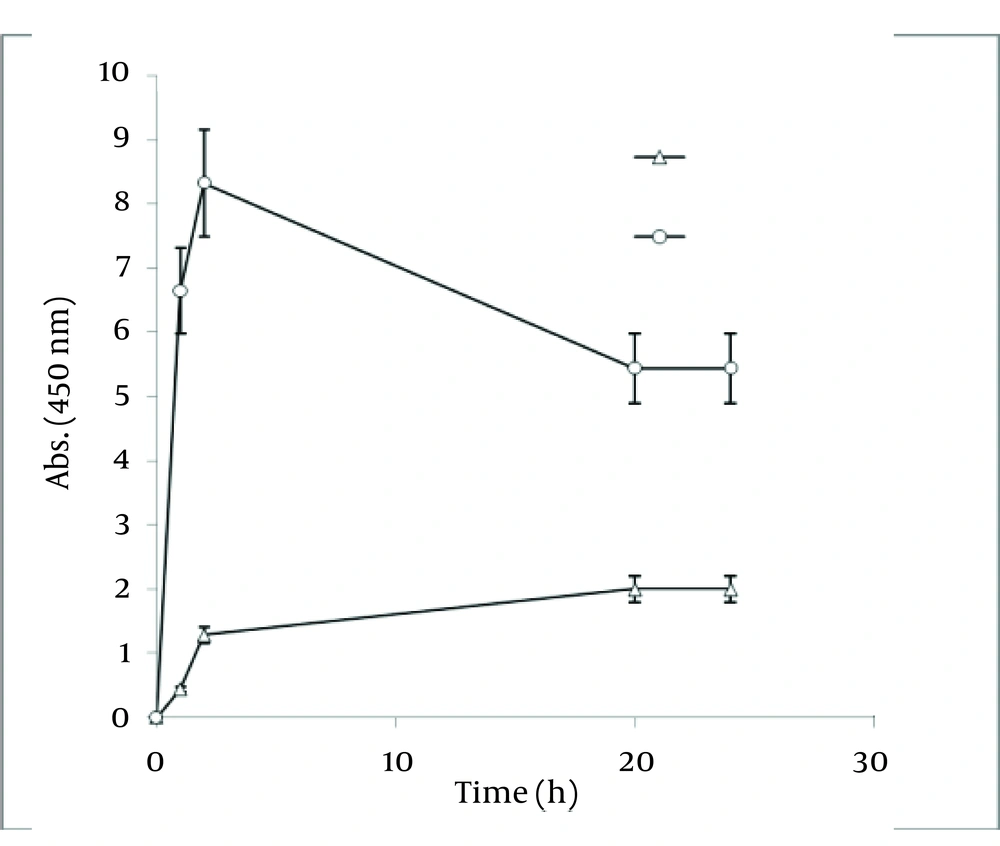
In order to extract plant phytochemicals, ethanol (70%) is a good solvent as it can dissolve and extricate biomolecules well and percolation is the most suitable technique to handle extraction process. Figure 2 reveals the time durations of silver nanoparticle formation with different walnut leaf ethanolic extract concentrations. By increasing the extract concentration, the number of produced nanoparticles was increased. The highest production of silver nanoparticles was observed at volume of 5 mL, 2 h after reaction initiation and then gradually decreased. In the case of boiled ethanolic extract, no absorbance was observed. It might be due to the destruction of reductive agents in the extract by boiling. In case of C. sinensis, the reaction rate was lower and increased to 168 h, and then nearly fixed (Figure 3). It appears that the stability of these nanoparticles were better than those produced by walnut. The maximum absorption of Ag nanoparticles occurred at 0.5 mL and higher concentrations of extract cut down the absorption. It might be interpreted that this concentration of extracts had negative effects on synthesis process. The boiled ethanolic extract had also no absorbance, which could be caused by the same reason as previously explained.
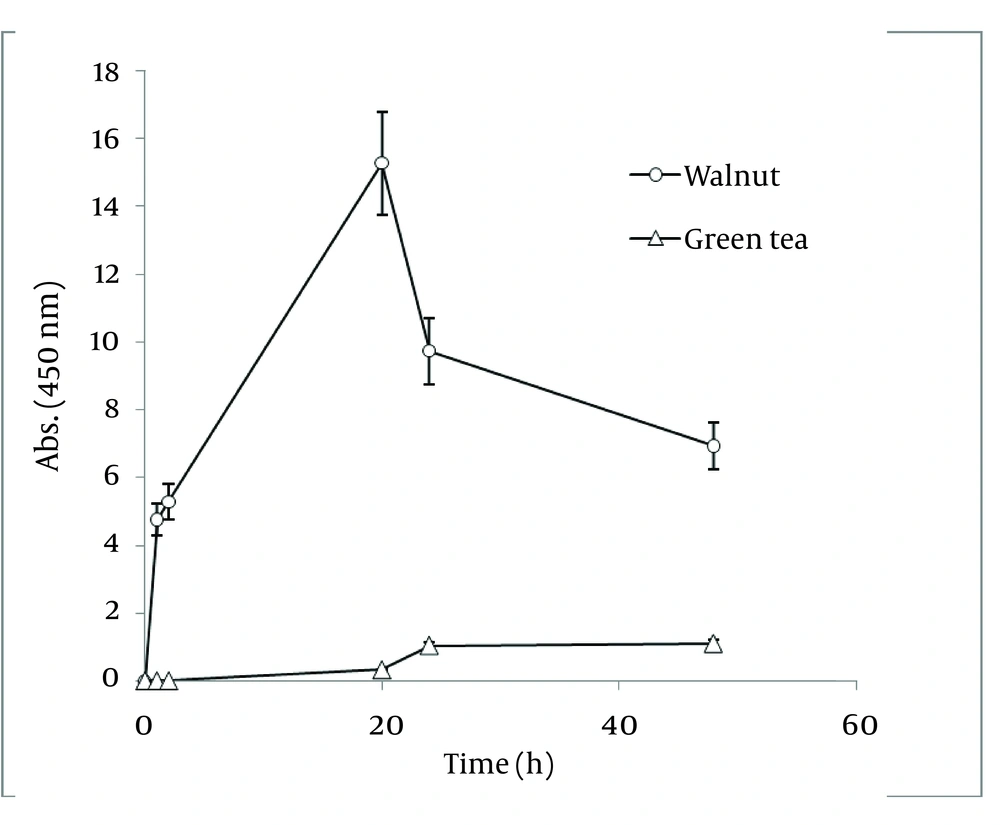
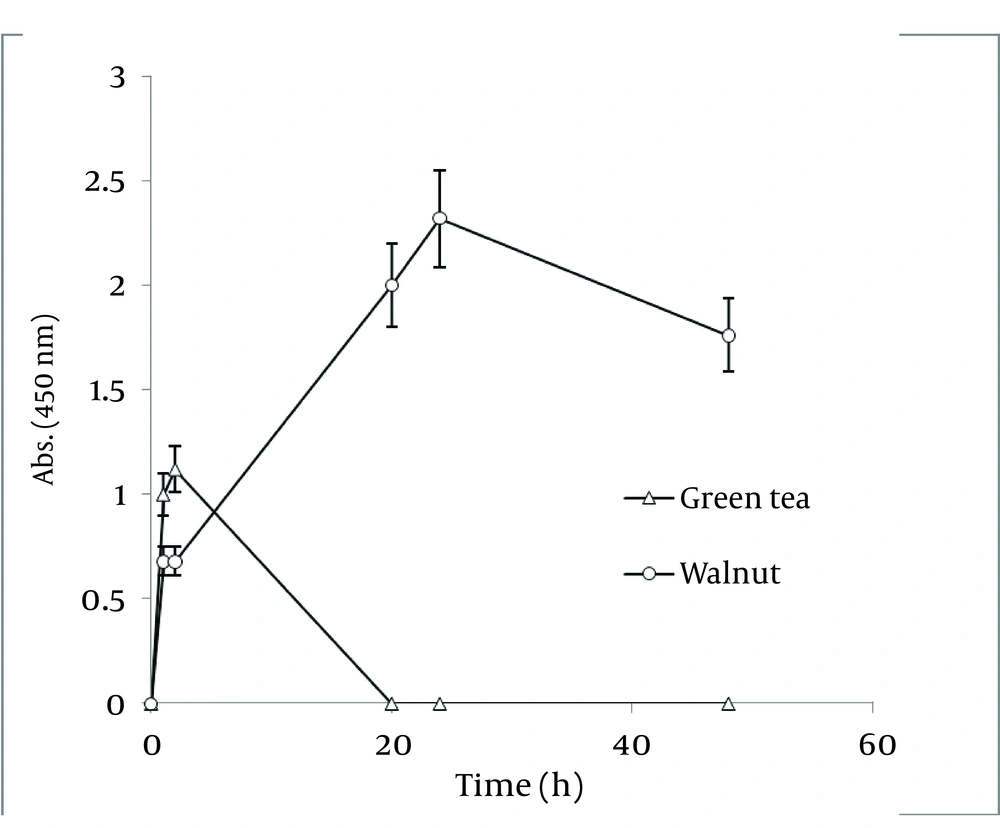
In order to compare nanoparticle production capability of the examined plants, the best results of their absorbance spectra has been revealed in Figure 4. According to the graph, J. regia has a much higher potential in nanoparticle synthesis than C. sinensis.
4.5. Nanosilver Particle Formation by Boiling Water Extract
To investigate the impact of plant boiling water extract (which has been used in many previous studies) (5-7, 9, 11, 21) upon nanoparticle production, different concentrations of Walnut leaf boiled water extract were studied. The absorbance in the reaction mixture with highest amount of extract increased to 24 h ( Figure 5 ). At a lower concentration, the absorbance was lower and declined after 2 h. In the case of C. sinensis, the reaction rate was faster than Walnut and increased during the first hour, then quickly reduced. In the reaction mixture with more extract concentration, the absorbance was higher ( Figure 6 ). By comparing the best absorbance spectra peaks of using two plant boiling water extracts, again the amount of nanoparticle production was very high in the case of Walnut, but C. sinensis had a faster reduction rate ( Figure 7 ).
4.6. Nanosilver Particle Formation by Plant Powder
In former studies, the powder of the dried plant was directly located in the substrate solution for producing metal nanoparticles (23, 33). Therefore, the effect of this method on nanoparticle synthesis should be evaluated as well.
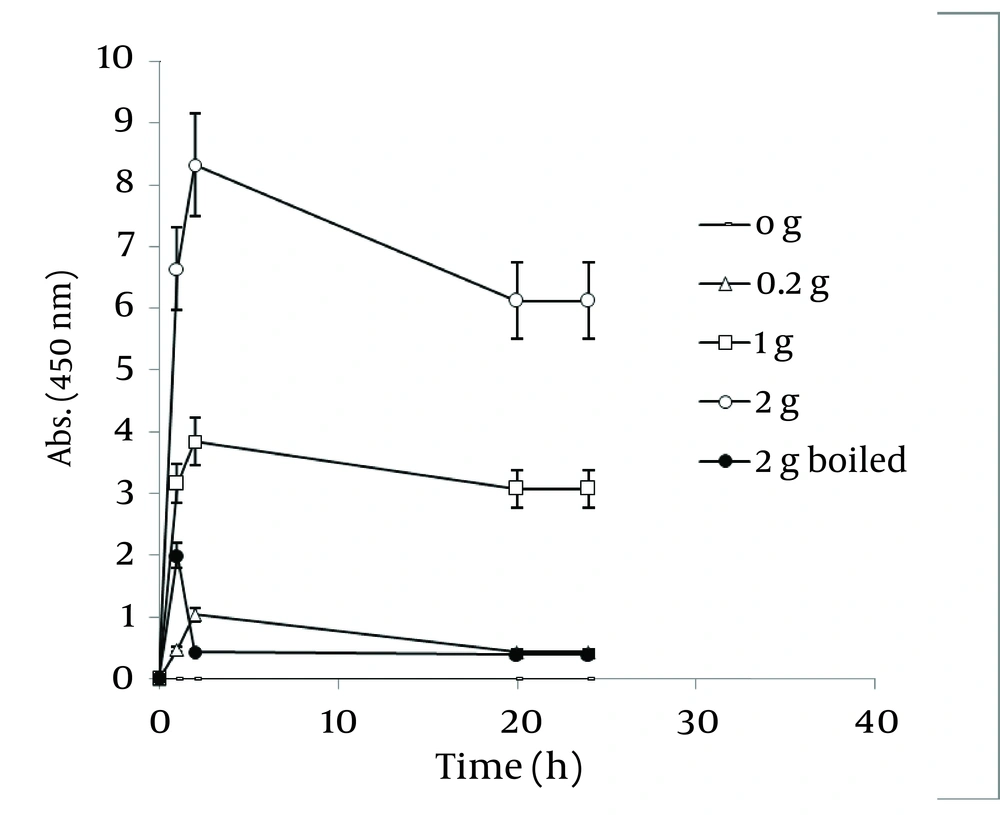
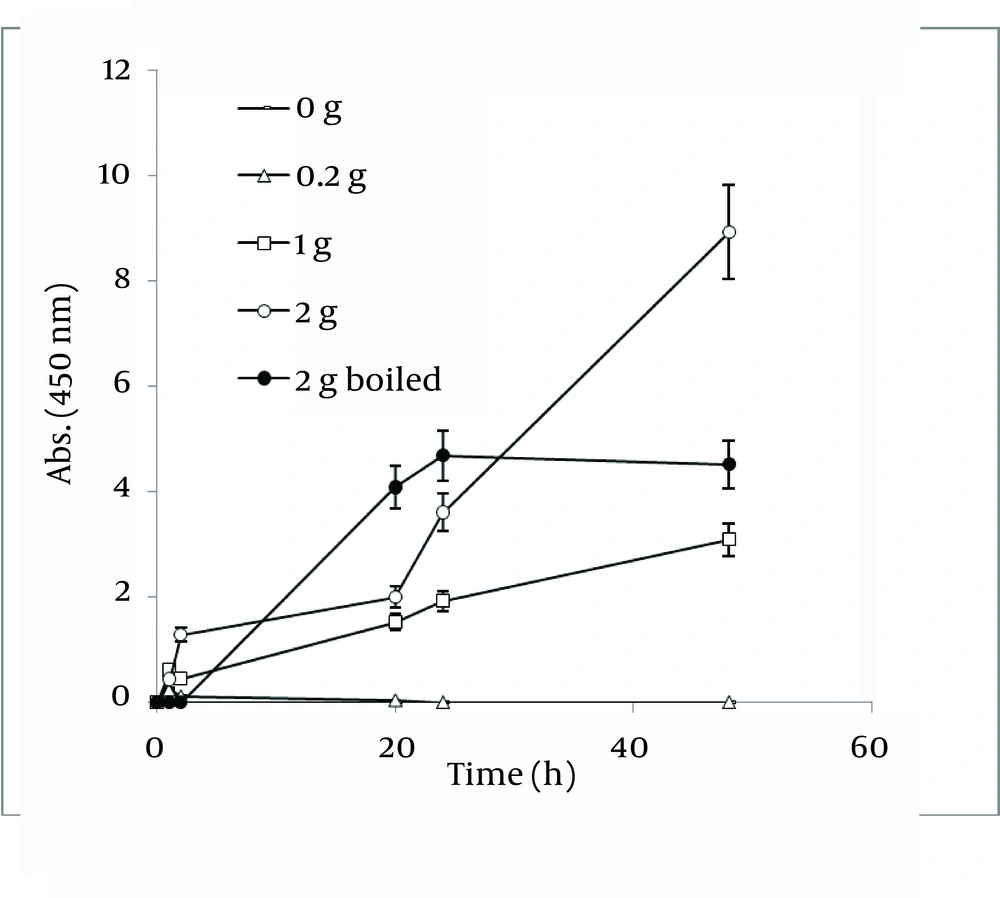
Figure 8 shows a straight relationship between the amount of Walnut leaf powder and Ag nanoparticle productivity. The absorbance intensified in the second hour and then decreased. The kinetic energy of produced nanoparticles by using several different powder amounts were the same. Boiling the resultant mixture containing the plant powder caused considerable a reduction in absorbance. In the case of Camellia sinensis, a dramatic increase in nanoparticle synthesis was observed versus using previous method (boiling water extract), but the rate of conversion to silver nanoparticles was less ( Figure 9 ). It might be interpreted that releasing reductive agents from the powder in the reaction mixture are dependent on the time span. By a gradual increase in amount of plant powder, the nanoparticle formation was increased. In the case of the boiled reaction mixture, the absorption stopped at 24 h. It could be argued that the reduction factors in the extract were limited after boiling. As presented in Figure 10 , the results of the absorbance spectra of Walnut and Tea (C. sinensis) powders were compared. Walnut consistently produced more nanoparticles than C. sinensis. The stability of both nanoparticles was similar.
4.7. Comparison of Three Methods Used for Nano-Silver Particle Formation
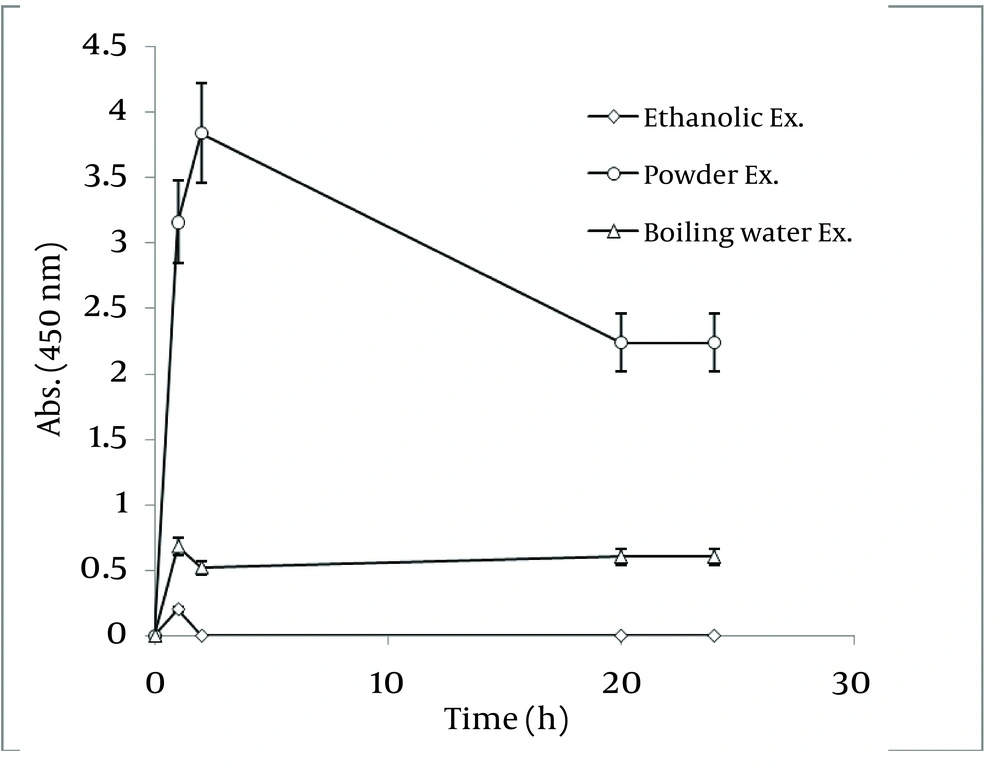
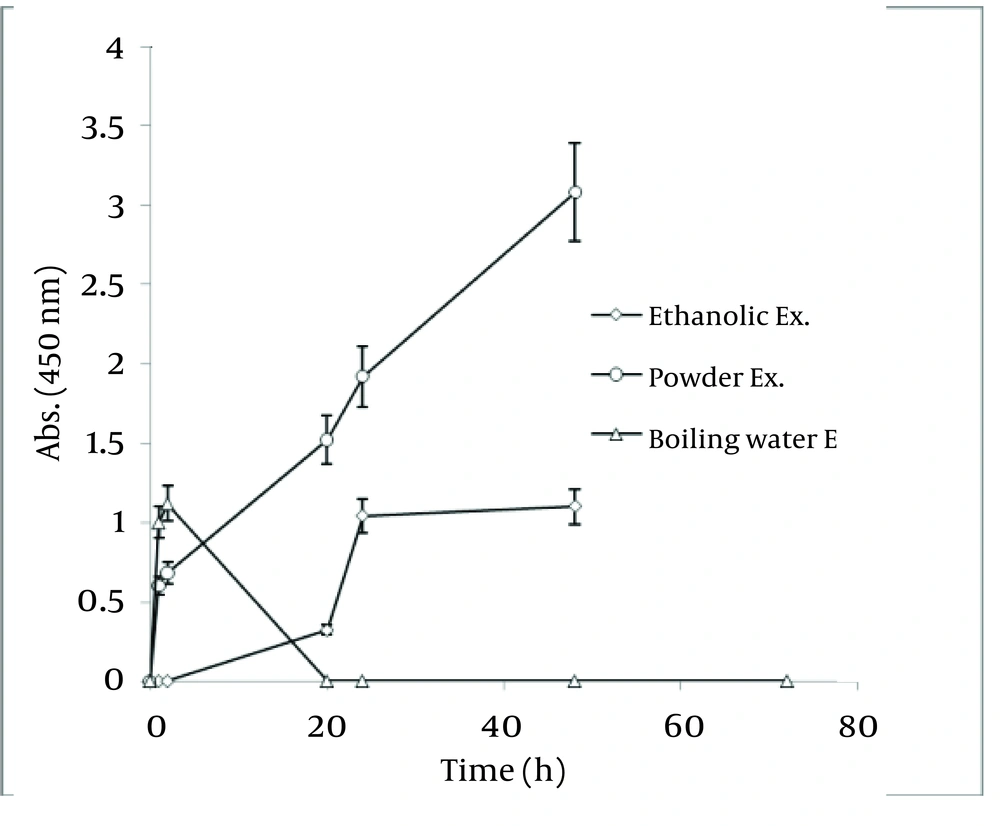
In order to realize the best route of employing Walnut leaf for Ag nanoparticle production, time intervals of sliver nanoparticle generation with three techniques of using Walnut leaf were revealed in Figure 11 . It should be noted that amount of J. regia powder used in these three methods was the same. Utilizing Walnut powder yielded much higher nanoparticles than two other methods, followed by ethanolic extract and boiling water extract. The stability of generated nanoparticles was similar in all of methods. In the case of C. sinensis, using the plant powder was also the most effective. Reaction rate of using boiling water extract was higher than powder and the absorbance increased in the first hour, then immediately declined ( Figure 12 ).
4.8. Analysis of Silver Nanoparticles with Transmission Electron Microscope (TEM)
Figure 13-A shows TEM micrograph of Ag nanoparticles produced by the reaction of AgNO3 solution with J. regia leaf powder, 2 hours following the start of the reaction. Silver nanoparticles were quasi- spherical, polydisperse (10-50 nm) and in aggregated form. Similar studies have been reported in Ag nanoparticle synthesis based on using plant powder. Bark powder of Cinnamon zeylanicum generated monodisperse Ag nanoparticles (31-40 nm) with spherical and rod shapes and in aggregated form (24), Euphorbia hirta produced cubic aggregated polydisperse silver nanoparticles in size of 40-50 nm (25) and using Cinnamomum camphora aggregated polydisperse nanoparticles (55-80 nm) with different shapes were synthesized (23), but in comparison with them, J. regia leaf powder yielded smaller dimensions of silver nanoparticles. Figure 13-B shows TEM micrograph of Ag nanoparticles produced by J. regia leaf ethanolic extract after 20 h. The nanoparticles were spherical, single (1-5 nm) and nearly monodisperse. The globules, which are seen in the graph, might be the fat molecules have been dissolved in the plant extract during ethanolic extraction and then after the addition of buffer and aqueous solutions, they were separated from the plant extract. Similar results based on using indirectly heated ethanolic extracts of Bryophyllum, Cyprus and Hydrilla was reported by Jha et al. and Fcc monodisperse Ag nanoparticles (2-5 nm) with no aggregation were produced (17).
5. Discussion
In the present study a new plant–Juglans regia debuted with an efficient production of silver nanoparticles. Moreover, different methods of employing this plant were compared for the first time. These trials illustrated that using the direct powder of Walnut produced more silver nanoparticles which is desirable from an economical and industrial perspective. However, using ethanolic extract of walnut leaf yielded non-aggregated nanoparticles with smaller dimensions. More details about its productivity were revealed, i.e. the influence of different extract concentrations and the effect of boiling the extract.