1. Background
Medicinal plants are useful and important sources for finding new antimicrobial agents. They contain active component that can be used in the treatment of many human diseases (1). The plant extracts have been prepared and proposed for use as antimicrobial substances. Recent estimates suggest that several thousands of plants have been known to have the potential of medicinal applications in various cultures (2). For 80% of the world population in Asia, Latin America, and Africa, use of herbal medicine as traditional health remedies is the most popular and is reported to have minimal side effect (3). Many studies indicate that in some plants, there are many substances such as peptides, some essential oils, phenols and water, ethanol, chloroform, methanol, and butanol soluble compounds (4). In Khuzestan, the southwest province of Iran, use of plants as folklore remedies for infections is a widespread culture. One of these plants, Urtica dioica, is commonly used in traditional medicine for curing different diseases. It has a long history of application as a medicine as well as a food source. This plant is widely scattered in Khuzestan fauna and is used by natives as medicinal plant. Concerning infectious diseases that are endemic in Khuzestan or occur as epidemics, finding alternate and natural resources for antibacterial agents is of great importance. Therefore, the present study was conducted in order to examine in vitro antibacterial potential of methanolic and ethanolic extracts of U. dioica. The results of this study can be helpful for treating infectious disease with natural products and fighting against drug resistant bacteria.
2. Objectives
The aim of the present study was to examine the antibacterial activity of U. dioica, its time-kill kinetics assay, and the structural changes caused by the extracts of this plant in target bacteria.
3. Materials and Methods
3.1. Plant Collection and Identification
The understudy plant was collected from Shahid Chamran University farmland in Khuzestan Province, Iran. These samples were identified and confirmed through comparison with existing herbarium in Department of Biology, Shahid Chamran University, Ahvaz, Iran.
3.2. Extract Preparation
The aerial parts of U. dioica were shade-dried at room temperature for ten days and then ground to a fine powder using electronic blender. Extraction was done from one gram of obtained powder using 10 mL of alcohol (ethanol or methanol, Merck, Germany) and distilled water solution (8:2 v/v), centrifugation at 3000 rpm for 15 minutes, and harvesting the supernatant. This process was repeated three times and solvents were evaporated by placing the yielded materials at room temperature for seven days (4, 5).
3.3. Bacterial Strains
A total of eight bacterial strains were tested including Bacillus cereus, Staphylococcus aureus, and Staphylococcus epidermidis as gram-positive and Salmonella typhi, Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa, and Proteus mirabilis as gram-negative species. These species were originally isolated from clinical specimens and identified according to standard phenotypic test. The antibiotic sensitivity patterns of these isolates were investigated according to Kirby-Bauer standard disc diffusion method.
3.4. Determination of Antibacterial Activity
Antibacterial activity of the ethanolic and methanolic extracts of U. dioica was surveyed by Kirby-Bauer disc diffusion method (6). A cell concentration of 105 CFU/mL of test bacteria, with reference to the McFarland turbidometry, were prepared by culturing in Muller Hinton Broth (MHB, Merck, Germany) medium and incubation at 37℃ for 22 hours (7). A lawn culture was prepared on Muller-Hinton Agar (MHA, Merck, Germany) using sterile cotton swab and allowed to remain in contact for one minute. Sterile filter paper discs (6.4 mm) were saturated by different effective doses of 1.5, 3, 6, 12, and 18 mg were placed on lawn cultures after solvent evaporation (2, 8). A saturated disc with ethanol and methanol was also prepared simultaneously and regarded as negative control. The plates were left at room temperature for one hour to allow the diffusion of extracts from the discs into the medium, and subsequently, the petri dishes were incubated at 37℃ for 24 hours. The inhibition zone around each disc was measured in mm and recorded.
3.5. Determination of Minimum Inhibitory Concentrations
The minimum inhibitory concentration (MIC) of the extracts was determined for the most sensitive bacterial species. For this purpose, macro-broth tube dilution assay was used. In this assay, twofold dilutions of extract were prepared in tubes containing 1 mL Muller-Hinton broth and bacterial suspension equal to 0.5 McFarland turbidity. These tubes were incubated at 37℃ for 24 hours. The first tube in above series with no sign of visible growth was considered as the MIC (8). This process was repeated three times.
3.6. Determination of Minimum Bactericidal Concentration
To determine the minimum bactericidal concentration (MBC), a loop full from those tubes, which did not show any visible growth in MIC assay, was cultured on MHA and incubated at 37℃ for 18 to 24 hours. The highest dilution that did not yield colony formation on MHA was considered as MBC (9).
3.7. Time-Kill Kinetic Study
The time-kill kinetic was studied by culturing one standard loop of the bacterial suspension (0.5 McFarland turbidity), that was treated with MBC concentration of extract on MHA, from zero through 36th hour after incubation. This was performed at one hour intervals for the first 18 hours and then at two hours intervals for the next 18 hours (8).
3.8. Scanning Electron Microscopy Analysis
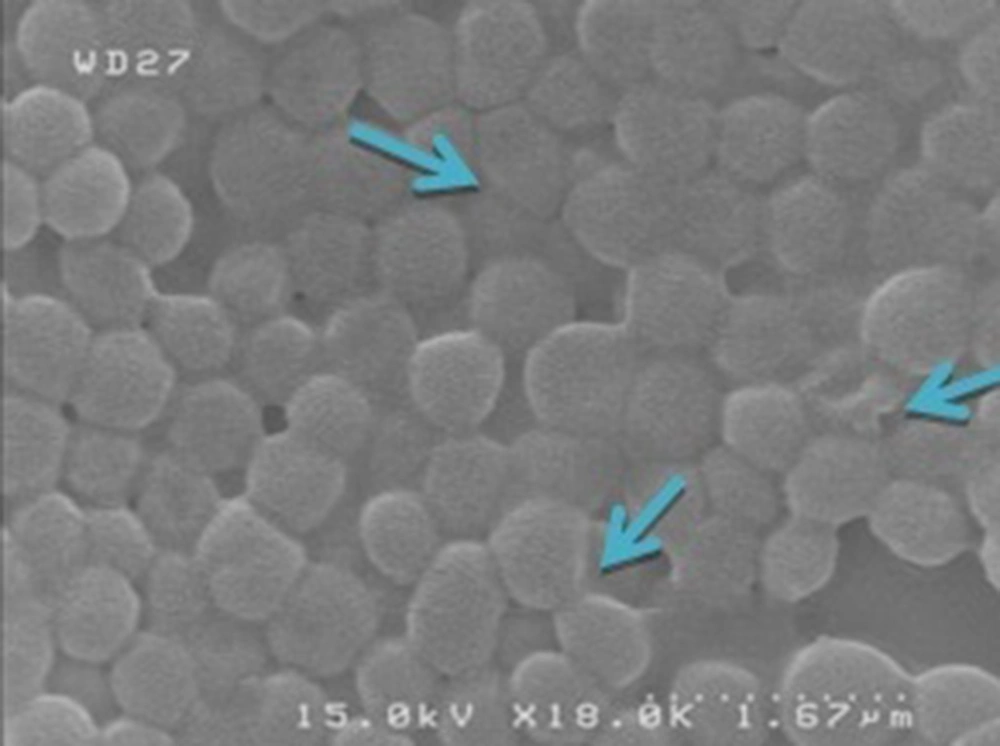
In order to find possible effect of active antibacterial substances of U. dioica alcoholic extracts, SEM (scanning electron microscopy) analysis was used. For this purpose, an antibacterial susceptibility test has been done with 12 mg effective dose from methanolic extract against S. epidermidis. After 24 hours, when halo of inhibition zone appeared around the disc, a sample was taken from the margin of the halo and was analyzed by SEM (Hitachi Japan S4160) after coating with gold.
4. Results
The antibacterial activity of ethanolic and methanolic extracts of U. dioica appeared as growth inhibition of tested bacteria. The results of the antimicrobial activity are given in the Tables 1 and 2. These results showed that these extracts were effective against four tested organisms including E. coli, S. epidermidis, B. cereus, and S. aureus. The highest activity (inhibition zone diameter about 18 mm) was demonstrated by the methanolic extract of U. dioica leaves against S. epidermidis while the lowest activity (inhibition zone diameter about 7 mm) was demonstrated by the ethanolic extract against B. cereus and S. aureus and by methanolic extract against B. cereus. On the other hand, the ethanolic and methanolic extracts were not active against S. Typhi, P. aeruginosa, K. pneumoniae, and P. mirabilis. The results of MIC and MBC of ethanolic and methanolic extracts for three bacterial species are shown in Table 3. Time-kill kinetic for methanolic extract of U. dioica was nine hours (Table 4). Figure 1 shows the structural changes of S. epidermidis following exposure to alcoholic extract. As we can found the round shape of this bacterium was changed to ovoid or irregular forms. Some of them were collapsed and appear to be somewhat indented. The size of the affected bacteria was also decreased and varied.
| Ethanolic Extract, mg | Methanolic Extract, mg | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 18 | 12 | 6 | 3 | 1.5 | 18 | 12 | 6 | 3 | 1.5 | |
| Gram positive | ||||||||||
| staphylococcus aureus | 9 | 8 | 8 | - | - | 10 | 8 | 7 | 7 | - |
| Bacillus cereus | 11 | 11 | 8 | 7 | - | 16 | 14 | 13 | 11 | 9 |
| Staphylococcus epidermidis | 17 | 15 | 12 | 9 | 9 | 18 | 14 | 13 | 12 | 10 |
| Gram negative | ||||||||||
| Escherichia coli | 16 | 13 | 10 | 9 | 8 | 14 | 11 | 10 | 9 | - |
Antibacterial Activity Results of Ethanolic and Methanolic Extracts of Urtica dioicaa
| Bacterial Species | Antibiotic Discb | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| NF | CB | NB | DX | OX | Van | Cef | Tet | Pen | |
| Gram positive | |||||||||
| staphylococcus aureus | R | 13 | 31 | 15 | R | 15 | 25 | 20 | 30 |
| Bacillus cereus | R | 7 | 18 | 18 | R | 15 | 12 | 14 | R |
| Staphylococcus epidermidis | R | 36 | 29 | 21 | R | -c | -c | -c | -c |
| Gram negative | |||||||||
| Escherichia coli | R | R | 17 | 11 | R | -c | -c | -c | -c |
| Klebsiella pneumoniae | R | R | 11 | R | -c | R | 13 | R | R |
| Pseudomonas aeruginosa | R | R | 16 | R | R | R | R | R | R |
| Salmonella typhi | R | 27 | 34 | 30 | R | -c | -c | -c | -c |
| Proteus mirabilis | -c | -c | -c | -c | -c | R | 19 | R | 15 |
Inhibition Zone of Standard Antibiotics on Tested Bacteriaa
| Extract | ||||
|---|---|---|---|---|
| Ethanolic | Methanolic | |||
| Bacterial Species | Staphylococcus epidermidis | Escherichia coli | staphylococcus aureus | Staphylococcus epidermidis |
| MIC | 10 | 40 | 40 | 10 |
| MBC | - | - | - | 20 |
Antibacterial Activity of the Ethanolic and Methanolic Extracts of Urtica dioica on Some Tested Bacteriaa
| Hours Extract | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 20 | 22 | 24 | 26 | 28 | 30 | 32 | 34 | 36 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methanolic | + | + | + | + | + | + | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
5. Discussion
Many researchers have reported the antibacterial activities of different plants (10, 11). In traditional therapy, U. dioica L. (Urticaceae family) have been known for some medicinal properties such as anti-inflammatory and antirheumatic (5, 12), cardiovascular (9), natriuretic, hypotensive, and acute diuretic effects (13), and stimulation of proliferation of human lymphocytes (14). It has also been used in the traditional therapy of hypertension (15). The effects of its nettle are also kindle in the therapy of the prostatic hyperplasia (16-18). In this study, a variety of bacterial strains was selected for investigating the antibacterial effects of ethanolic and methanolic extracts of U. dioica leaves. The results of this study showed that the hydroalcoholic extracts of this plant have wide range of antimicrobial activity against both tested gram-positive and gram-negative bacteria, which is comparable to the effect of standard antibiotics. The results demonstrated that gram-negative bacteria including S. typhi, K. pneumoniae, P. aeruginosa, and P. mirabilis were resistant to all of the concentrations of both extracts. Their resistance can be due to unique structure of gram-negative bacteria. They have an outer membrane envelope that act as a barrier to the entrance of some substances and their porins determine the type and size of those substances that can reach to their cytoplasm. Furthermore, some of these resistant species can produce polysaccharide capsule that prevent the entrance of some substances to the cytoplasm. Considerable antibacterial activity against gram-positive species suggests that the extracts of U. dioica can be selective agents for treatment of infections caused by these organisms. Notably, excellent inhibition against Staphylococcus species can be a hope for discovering new natural antibacterial agent against coagulase-positive and coagulase-negative staphylococci, especially methicillin-resistant S. aureus (MRSA) and vancomycin-intermediate S. aureus (VISA) strains. Today, resistant S. aureus (MRSA, VISA, and vancomycin-resistant S. aureus [VRSA]) strains are a serious threat to humans, especially for nosocomial infections. These results showed that the target site of active constituents of U. dioica was bacterial cell wall. As the peptidoglycan synthesis and cell shape of bacteria is dependent to penicillin-binding proteins (PBPs), it can be concluded that these antibacterial substances inhibited these proteins and consequently could disrupt the integrity of peptidoglycans. These types of antibacterial compounds have two important superiorities over the others. First, they have bactericidal effect and recurrence of the infection due to the persistence of pathogen will be prevented. Second and most importantly, these agents affect cell wall and with regard to the uniqueness of this structure, these compounds can be regarded as safe antibacterial agent with no or less side effects on eukaryotic cells. Furthermore, the cell wall has a significant role in maintaining high internal pressure of bacterium. Those antibacterial agents that can affect this structure are suitable for killing bacteria (19).
It is known that many factors affect antibacterial activity. Therefore, we believe that the bacterial inhibition or killing can vary based on the plant extract, the used solvent, and the tested organisms. However, to get maximum antibacterial activity, both the solvent and the extraction system influence the final results. The results of this study suggest that the extracts of U. dioica can be used as antibacterial substance against some bacterial species such as S. aureus, E. coli, and S. epidermidis infections. This can be hopeful for finding new natural antibiotics for fighting infections. Dogruoz et al. surveyed antibacterial activity of ten aqueous and one ethanolic extracts from medicinal plants used in turkey. They found that U. dioica had no significant effect on E. coli, S. epidermidis, S. aureus, P. aeruginosa, and K. pneumoniae (20). Gulcin et al. discovered the antioxidant and antimicrobial properties of aqueous extract of nettle (U. dioica L.). As a result, it showed antimicrobial activity against nine microorganisms such as E. coli, P. mirabilis, and S. aureus (21). Finally, based on these results, it can be suggested that U. dioica alcoholic extracts target bacterial cell wall and can kill their target bacteria. Therefore, this plant is a favorable option in discovering new bactericide antibiotics, especially against Staphylococcus species.