1. Background
Leishmaniasis is an infection caused by the protozoan parasite Leishmania spp. The organism is transmitted to humans by the sandfly bite. Humans are usually accidental hosts of these 2 mm long flies; natural hosts include a variety of rodents, small mammals, and dogs. The disease is geographically and ecologically widespread, occurring in tropical and subtropical regions on all continents except Australia (1). A patient with leishmaniasis may be presented with one of three quite distinct clinical syndromes: visceral, cutaneous, or mucosal. Disease progression is dependent on both the species of Leishmania involved and the genetics and immune status of the host (2).
Cutaneous leishmaniasis (CL) is defined as a skin ulcer at the site of the sandfly bite and generally heals spontaneously with a scar within 3 to 6 months (1, 3). Cutaneous leishmaniasis requires treatment only when the lesions are large, multiple, disfiguring, or over a joint (3). Pentavalent antimonial have been used since the 1940s. It needs multiple injections, are painful, not tolerated by most of the patients; moreover, they are not always effective and resistance is reported too (4, 5). The World Health Organization/tropical disease research program emphasizes on the development of alternative treatments for CL. Paromomycin sulfate (PMS), a very hydrophilic antibiotic, is an aminoglycoside antibiotic produced by Streptomyces rimosus. The PMS was reported to show anti-leishmania activity in the 1960s and since then promising results were seen in clinical trials against both CL and VL (6-8). Recently, the parental formulation of PMS has been approved for the treatment of VL. Nevertheless, systemic use of PMS might cause nephrotoxicity, thus introducing a topical form could be advantageous. Although the topical approach for the treatment of CL is attractive, there are some aspects that make the treatment more difficult, the most important one is that Leishmania amastigotes lie deep in the dermal layer making penetration and achieving local therapeutic concentration difficult (9).
PMS in the conventional ointment and cream bases is not that effective because of its relatively large molecular size (C23H45N5O14. H2SO4, MW = 713.71), high water-solubility, and oligosaccharide nature, which cannot penetrate through the stratum corneum of the skin. Topically applied agents must be able to pass through the stratum corneum to be active against parasites inside the macrophages which lie deep in the dermal layer of skin and multiplied within the phagolysosome of the macrophage with a pH of 4.5 to 5.0 (7, 10). It is well known that macrophages internalize particulate carriers by the process of endocytosis and may act as a secondary drug depot, thus helping the localized delivery of the drug at the infected site (11). This property of liposome is an advantage in the treatment of intracellular infections involving this type of cells.
Jaafari et al. (12) investigated the antileishmanial effects of topical liposomal PMS major-infected BALB/c mice. They treated the lesions topically with 50 mg formulation twice a day for 4 weeks and reported that every mouse treated with liposomal PMS was completely cured at the end of the treatment, and no relapse was seen 4 weeks after the treatment. The spleen parasite burden was significantly lower in mice treated with liposomal PMS than in mice treated with PBS or control empty liposomes (P < 0.001). The treatment may have had an effect on the overall course of the infection and might be the reason for the lower parasite burdens in the spleens of LPMF-treated mice. Furthermore, since some of these PMS liposomes are very small, they can act as transdermal vehicles and pass from the epidermis intact and reach the bloodstream in the dermis. When liposomes are in the blood, spleen macrophages and Kupffer cells in the liver are the main cells that phagocytize the liposomal particles.
2. Objectives
This study aimed to evaluate probable nephrotoxicity and hepatotoxicity of topically administered PMS liposomes (same formulation tested in Jaafari et al. study).
3. Materials and Methods
3.1. Animals
Male Wistar rats (6-7 weeks old) were purchased from the center of animals’ maintenance and reproduction of Ahvaz Jundishapur University of Medical Sciences (AJUMS). They were housed in animal house of the School of Pharmacy in the well ventilated room, temperature of 21°C, 12 hours natural light and 12 hours darkness, with free access to tap water and dry rat pellet. The animal experiments were carried out according to Ethics Committee Acts of the AJUMS.
3.2. Chemicals
Soybean phosphatidylcholine (SPC) was obtained from Avanti polar lipids (Alabaster, AL). PM, propylparaben (PP), methylparaben (MP), cholesterol, and HEPES (4-(2-Hydroxyethyl) piperazine-1-ethanesulfonic acid or N-(2-Hydroxyethyl) piperazine-N'-(2-ethanesulfonic acid) were purchased from Sigma. Vitamin E was purchased from Merck (Darmstadt, Germany).
3.3. Preparation of Formulations Containing Paromomycin Sulfate
Liposomes containing PM were prepared by the fusion method. In brief, the lipid components consisted of SPC (15%), cholesterol (2%), propylene glycol (7%), vitamin E (0. 3%), MP (0. 1%), and PP (0.02%); these components were melted at about 75°C (lipid melts). HEPES buffer (10 mM, pH = 7.0) and PMS (10%) were heated separately and added up to 100% to the previously heated melted lipids, and the mixture was vigorously vortexed and allowed to cool down at room temperature. The final products were then homogenized with a homogenizer (Ultra-Turrax IKA T50; IKA Werke GmbH & Co. KG, Staufen, Germany) for 5 min at 5,000 rpm. The same procedure was used for the preparation of control empty liposomes, except that PMS portion was omitted (12).
3.4. In Vivo Experiment, Preparation of Animals for Topical Administration
From the formulated sample, 50 μL twice a day were topically administered to 9 groups of rats consisting of 6 rats in each group that were studied in 3 time periods of 10, 20, and 30 days. Three groups were placed in each time period. Group A as the control group, did not receive any medication; group B received nanoliposome without paromomycin sulfate and group C received nanoliposomal formulations containing paromomycin sulfate. At the end of the experiment, the rats were weighed and blood samples collected through a carotid vein under ether anesthesia. The blood sample was allowed to clot, and serum was separated and used to determine the enzyme activities such as glutamate oxaloacetate transaminase (SGOT), glutamate pyruvic transaminase (SGPT), alkaline phosphatase (ALT), BUN, and creatinine.
3.5. Histopathology examination
After blood collection, kidney and liver were removed from the body for histopathology examination.
3.6. Statistical Analysis
The results of liver function and kidney were calculated as mean and standard error of the mean (mean ± SEM); differences between groups were tested using analysis of variance (ANOVA) followed by the test method. Furthermore, Tukey post hoc was performed, and the data analysis method was used to test Tukey. P ≤ 0.05 was considered significant.
4. Results
4.1. The Results of Liver and Renal Function Tests
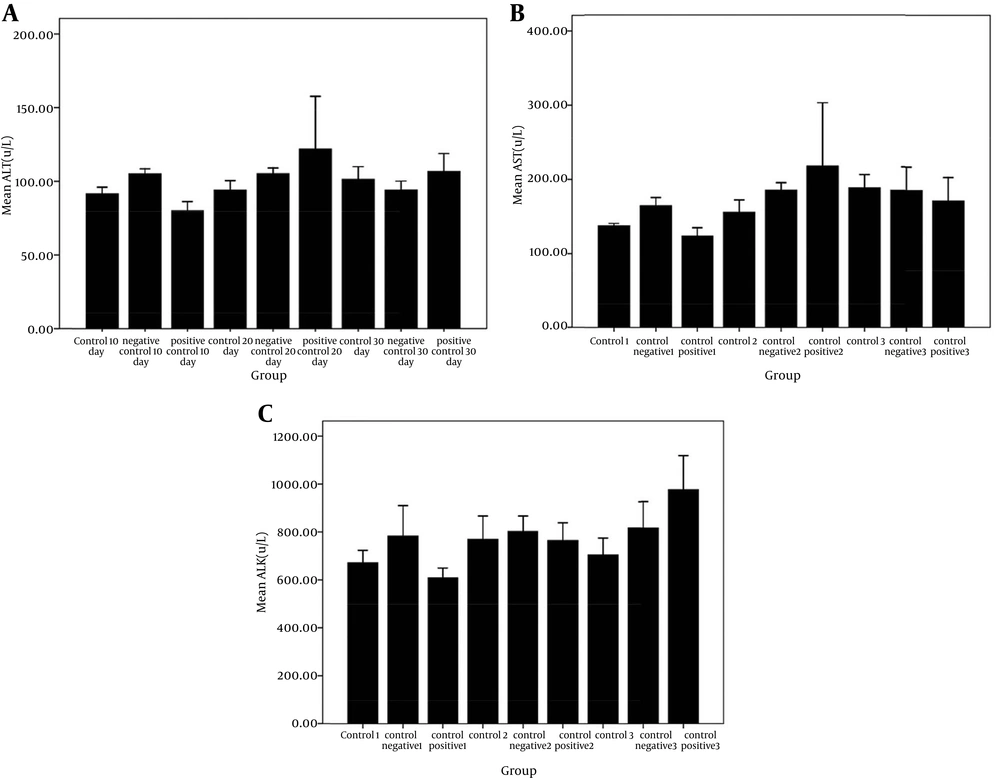
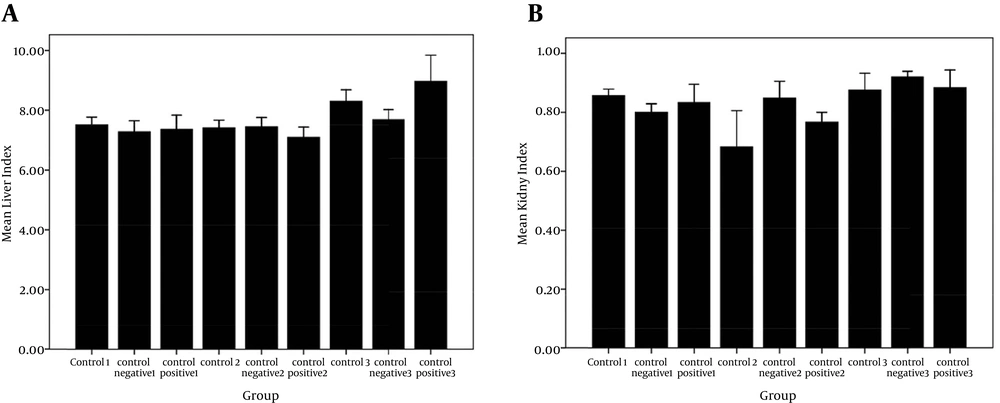
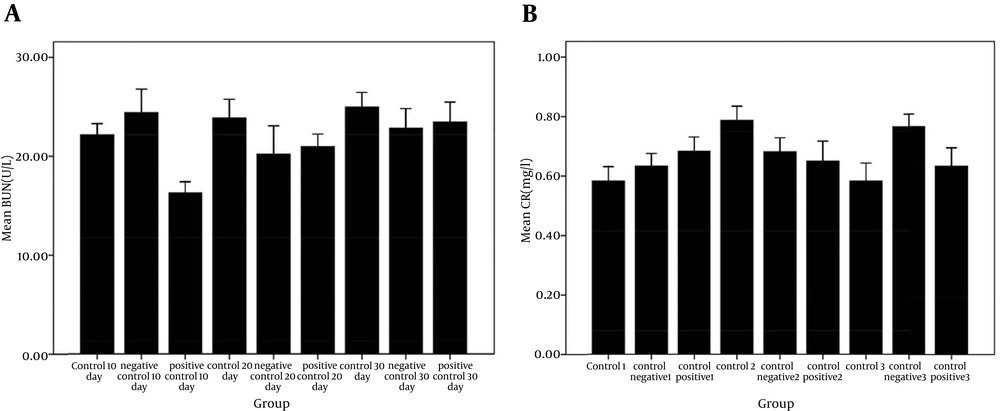
The results of the liver tests showed that the groups receiving liposomal formulations containing paromomycin sulfate during three periods of 10, 20, 30 days compared with the control, and negative control groups, no significant difference was seen in the amount of ALT (Figure 1 A), AST (Figure 1B), and ALK (Figure 1 C). These tests did not show any liver damage. Also, significant differences in the liver weight index (Figure 2 A) were not observed in the study groups compared to the control and control positive groups. The results of kidney tests showed that there was no significant difference in the BUN (Figure 3 A) and serum creatinine (Figure 3 B) between groups receiving topical application of liposomal formulations containing paromomycin sulfate (examined of three periods 10, 20, 30 days) and the control and control positive groups. Also, no significant difference in the kidney weight index (Figure 2 B) was observed in the study groups compared with the control and control negative groups.
A, comparison of liver enzyme ALT level in serum of control and negative control groups with positive group receiving PMS during three periods of 10, 20, and 30 days in the rats. No significant difference was seen among the groups (P > 0.05); B, comparison of liver enzyme AST level in serum of control and negative control groups with positive group receiving PMS during three periods of 10, 20, and 30 days in the rats. No significant difference was seen among the groups (P > 0.05); C, comparison of liver enzyme ALK level in serum of control and negative control groups with positive group receiving PMS in the during three periods of 10, 20, 30 days in the rats. No significant difference was seen among the groups (P > 0.05).
A, comparison of liver index in control and negative control groups with positive group receiving PMS during three periods of 10, 20, and 30 days in the rats. No significant difference was seen between groups (P > 0.05); B, comparison of kidney index in control and negative control groups with positive group receiving PMS during three periods of 10, 20, and 30 days in the rats. No Significant difference was seen between groups (P > 0.05).
A, comparison of BUN level in serum of control and negative control groups with positive group receiving PMS during three periods times of 10, 20, and 30 days in the rats. Data are shown as mean ± SEM. No significant difference was seen between groups (P > 0.05); B, comparison of Cr level in serum of control and negative control groups and positive group receiving PMS during three periods of 10, 20, and 30 days in the rats. No Significant difference was seen between groups (P > 0.05).
4.2. Histopathological Studies
Nanoliposomal formulation containing paromomycin sulfate to assess potential effects on the liver and kidneys of rats after H&E staining: histopathological changes were evaluated in the rats; kidney and liver in the positive control group were compared with control and negative control groups.
4.2.1. The Results of Histopathological Examination of Liver
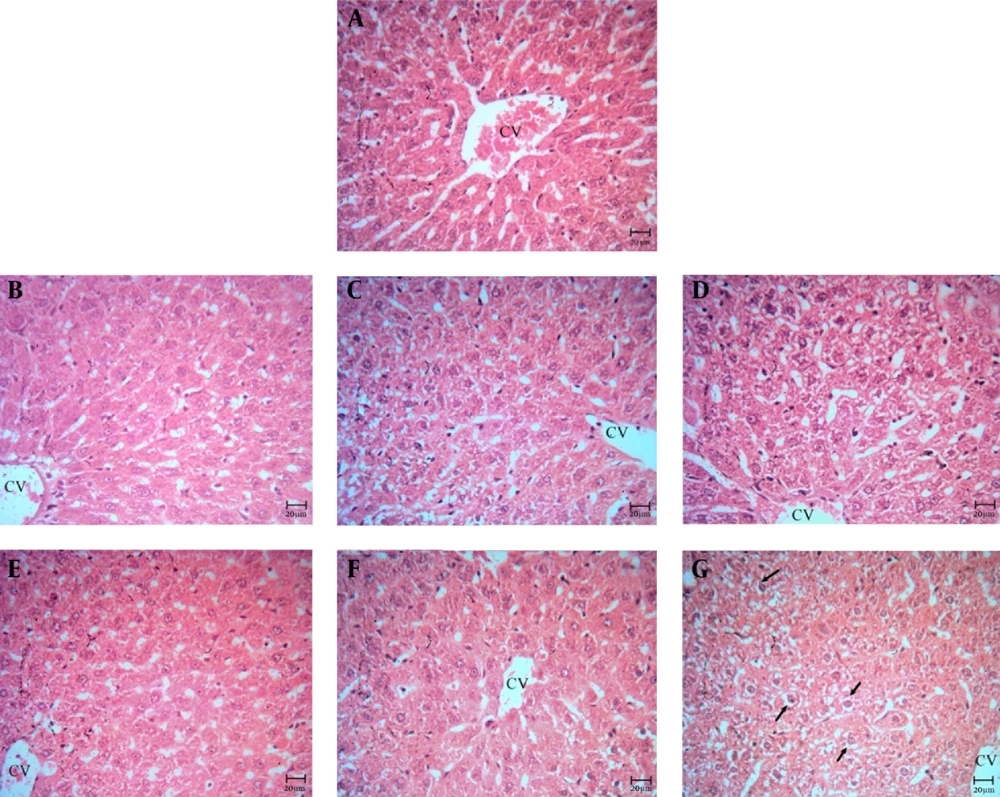
Livers of the animals in control and negative control groups, which were sacrificed after 10, 20 and 30 days, showed a normal structure (Figures 1-4). Also, rats in the positive control group, which were euthanized in 10 and 20 days, had normal livers (Figures 5E and 5F). Histopathological inspection of the positive control group that was euthanized after 30 days, revealed severe cell swelling in hepatocytes (Figure 5G). Vacant spaces in hepatocytes were diffused, and enlarged.
A, control group; B, negative control (10 days); C, negative control (20 days); D, negative control (30 days); E, positive control (10 days); F, positive control (20 days). Note to the normal structure of liver (Figure 4 A-F) and hepatocytes which are located around the central vein (CV); G, positive control (30 days). Cell swelling in hepatocytes is obvious (arrows).
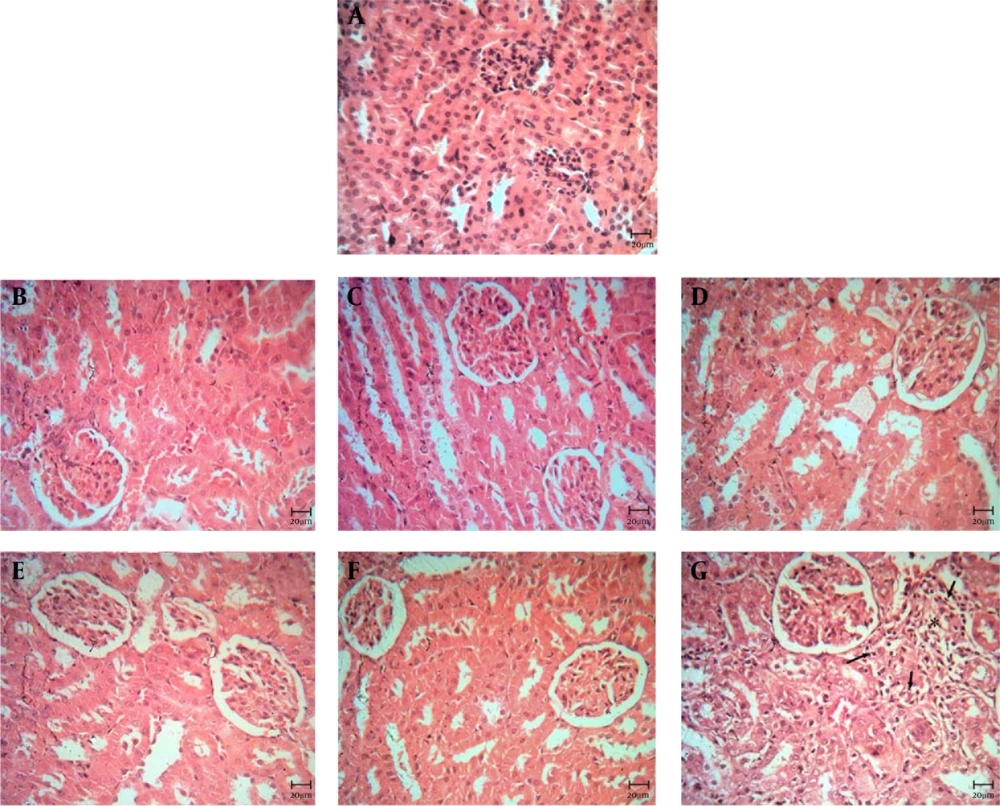
4.3. The Results of Histopathological Examination of Kidney
Histopathological study of kidney in control and negative control groups showed a normal structure (Figures 5A-D). Also, in the positive control group, the animals' kidneys were sacrificed at 10 and 20 days, the kidneys' structure was normal (Figures 5E and F). In 30-day group, mild tubular necrosis was seen (Figure 5G).
A, control group; B, negative control (10 days); C, negative control (20 days); D, negative control (30 days); E, positive control (10 days); F, positive control (20 days). Note to the normal structure of the kidney (figures 5A-F); G, positive control (30 days). Mild tubular necrosis was seen (star).
5. Discussion
The liver is known as one of the main organisms in the human's body and the key place for excretion and metabolism, which plays various roles in synthetizing proteins, detoxification, and producing the biochemical compounds essential for digestion system. Liver diseases are considered as one of the major causes of morbidity and mortality in human in the world; furthermore, it seems that hepatotoxicity due to drugs is the most common cause of the death. The damaged structural integrity of the liver increases serum levels of transaminases (AST and ALT) and ALP because they are usually found in the cytoplasm and released into the circulation system during cellular damage (13).
Nephrotoxicity due to the drugs is usually associated with their accumulation in the renal cortex, their affinity to kidneys and kinetics of the drug trapping process (14). Aminoglycoside nephrotoxicity is functionally expressed by the reduced capacity of the urinary concentration, lysosomal enzymuria, reduced ammonium excretion, mild glycosuria, tubular proteinuria, and dropping glomerular filtration rate (GFR). About 8% to 26% of patients receiving aminoglycosides for more than 7 to 10 days developed a mild renal injury, which was nearly at all times reversible (14, 15). Gentamicin, which is a typical aminoglycoside antibiotic, is extensively used in the clinical practices. Nephrotoxicity induced by gentamicin is characterized by direct tubular necrosis, without morphological changes in glomerular structures (14).
The use of liposomes in the pharmaceutical industries caused a considerable improvement, particularly through controlling and increasing the drug level in the body and its side effects and the dosage reduction (16). In general, liposomal formulation compared with the normal formulation increases the penetration of drugs into the skin. Also, the drug-delivery systems would be more stable and targeted (9). The concentrations of phospholipid and the existence of polyunsaturated fatty acids in the liposomes are among the effective factors on the severity of the penetration of drugs into the skin. Liposomes not only can pass through subcutaneous tissue, but also they can enter the systemic blood stream. One of the reasons for the application of the liposome in topical medication formulation is that phospholipid existing in the structure of the liposomes can penetrate the lipid compounds of the skin and can be released on the inside of the subcutaneous tissue, and as a result of interaction with them and the combination of liposomal phospholipid with lipids contained in the membranous skin layers, the membrane fluidity of the skin is impaired, and skin defensive characteristics reduce.
Penetration of hydrophilic drugs into the skin is difficult, because the nature of the skin is lipophilic (9). Since the liposomes size in the liposomal formulation containing paromomycin sulfate is very fine, they may enter the bloodstream through the epidermis by passing the derma. These particles when enter the bloodstream are phagocytosed by Kupffer cells in the liver and splenic macrophage (12). Aminoglycosides cause proximal tubular injury and necrosis and renal dysfunction. About 10% of kidney problems are associated with the use of these drugs (15). Paromomycin like all aminoglycoside antibiotics inhibits the biosynthesis of proteins in the living organisms. Its antileishmanial activity was discovered in 1960. Also, its antileishmanial activity was reported in the clinical tests versus both forms of visceral leishmaniasis (VL) and cutaneous leishmaniasis (CL).
In Jaafari et al. (12) in vivo study, results in BALB/c mice infected with L. major showed that using topical formulations containing paromomycin sulfate induced a significantly smaller size lesion and lower spleen parasite burden than the control groups and PMS cream. It is speculated that after topical application of PMS formulations, at least some of the vesicles, especially those with smaller than 100 nm, pass through subcutaneous tissue of intact skin and reach the bloodstream.
In this study, topical PMS liposomes prepared by the fusion method plus homogenization provided liposomes of submicron sizes. Analysis of the particle size distribution showed that the average size of LPMFs was less than 100 nm. To comment on the liver toxicity, tests for ALK, AST, ALT showed nanoliposome formulations containing paromomycin sulfate with 10% concentration in the group study had no significant difference in this enzyme compared with the control group and negative control groups during the three time periods, (10,20, and 30 days) and this indicates no drug hepatotoxicity in rats. On the contrary, increase in enzymes AST, ALK, and ALT particularly indicates liver toxicity. It can be concluded that nanoliposomal formulation of paromomycin sulfate after topical application and systemic absorption has no toxic effects on liver cells.
Also for assessing nephrotoxicity, evaluation of creatinine and BUN levels showed that liposomal formulation of paromomycin sulfate did not make significant changes in BUN and creatinine levels. BUN and creatinine serum are the most common lab indicators of renal function. BUN like creatinine is directly excreted by the kidneys. Renal normal function and normal serum creatinine levels should remain constant. Therefore, we can talk with more certainty about the effect of nanoliposomal formulations of paromomycin in kidney and liver of rats.
Furthermore, examination of histopathological effects on the liver and kidney are necessary. The pathological result of the liver showed that the negative control group compared with the control group in periods of 10, 20, and 30 days, had a normal structure. Positive control group during the periods of 10 and 20 days did not show changes in the liver compared with the control group and negative control groups. In 30-day period, in the positive group compared with the control and control negative groups, changes as cell swelling were observed, which were reversible and negligible. Histopathological results of the kidney showed that structure of negative control and positive control groups compared with control group had not changed in the periods of 10 and 20 days is normal. The group receiving nanoliposomal paromomycin sulfate in the 30-day period was compared with the control and negative control groups and mild tubular necrosis was seen in the kidney.
Considering that previous studies have approved the effectiveness of the formulation of nanoliposomal formulation containing paromomycin sulfate on leishmania as well as topical use of this formulation cured the wounds relatively and reduced the microbial load of the spleen due to the systemic absorption of part of the drug; therefore, the possibility existed that it causes toxicity. Accordingly, this study examined renal and hepatic toxicity caused by topical formulation of nanoliposomal formulation containing paromomycin. The results showed that the short-term use of nanoliposomal topical formulations containing paromomycin sulfate formulations for treatment of the CL has not created certain toxicity in kidney and liver, but its long-term use leads to mild renal tubular necrosis and reversible cell swelling in the liver. Therefore, it should be used with caution during long-term treatments.