1. Background
Acrylamide (ACR) is a low-molecular-weight vinylic compound used to produce polyacrylamides. Polymers are used in different industries like wastewater treatment, soil coagulation, dye synthesis and gel chromatography in laboratories. ACR monomer is a potent neurotoxin and animal carcinogen; however, the polymer is not toxic (1-3). ACR has been known as an occupational hazard for decades (4, 5). Moreover it has been found to form in fried and baked starchy foods during cooking (6). Evidence has shown that ACR is a potent neurotoxicant in both humans and animals. Low-level exposure to ACR causes skeletal muscle weakness, ataxia, myalgia and weight loss (7). Beside neurotoxicity, ACR can induce reproductive toxicity, genotoxicity and carcinogenicity (8-10). It was shown that ACR subchronic exposure could affect the expression of death-related proteins in the central and peripheral nervous systems (11). Moreover, ACR increased intracellular reactive oxygen species (ROS) in PC12 cells, which played an important role in ACR induced cytotoxicity (12). Growing evidence has shown that enhancement of lipid peroxidation and impairment of antioxidative capacity in the central and peripheral nervous systems are considered as mechanisms of ACR-induced neuropathy (13).
Recently, there has been increasing interest in potential human health benefits of natural compounds. Green tea is made up of leaves of the Camellia sinensis Theaceae. Many of the profitable properties of green tea are related to the activities of epigallocatechin gallate (EGCG), the major compound of green tea catechins (14).
Green tea and its constituents are widely evaluated for their pharmacological activities such as anti-cancer (15, 16), anti-obesity (17, 18), anti-atherosclerotic (19), anti-diabetic (20), hepatoprotective (21), anti-bacterial and anti-viral effects (14). Green tea and its components have shown antioxidant effects in different in vitro and in vivo studies (22, 23).
Antioxidants have been reported to protect neurons from neural loss by reducing ROS-mediated reactions (24-27).
2. Objectives
In the present study, possible protective effect of GTAE on ACR-induced neurotoxicity was evaluated in both in vivo and in PC12 cells as a suitable in vitro model for evaluation of neurotoxicity.
3. Materials and Methods
3.1. Chemicals
RPMI 1640 and FBS were purchased from Gibco. (4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium (MTT) and ACR were obtained from Sigma (USA) and Merck (Germany).
3.2. Plant Materials and Preparation of GTAE
Green tea leaves (Place of origin: North of Iran) were collected. The leaves were powdered using a milling machine. Fifty grams of green tea powder was macerated in 500 mL of boiling water for 15 minutes. The extract was centrifuged at 3000 × g for 7 minutes and the supernatants were pooled and then lyophilized.
3.3. Determination of Total Polyphenol Content of Green Tea Extracts
The amount of polyphenol was measured by a photometric Folin-Ciocalteu assay using the standard gallic acid calibration curve. The method was based on the reduction of phosphotungstic acid in alkaline solution to phosphotungstic blue (28). Briefly, one mL of GTAE was mixed with one mL of three-fold-diluted Folin-Ciocalteu phenol reagent. Two milliliters of 35% sodium carbonate solution was added to the mixture, which was then shaken thoroughly and diluted to 6 mL by adding 2 mL of water. The mixture was incubated for 30 minutes and blue color formed was measured at 700 nm using a spectrophotometer. A calibration curve of gallic acid was prepared and the results were expressed as mg gallic acid equivalents per gram of dried weight of the tea.
3.4. Cell Culture
PC12 cells were obtained from Pasteur Institute (Tehran, Iran). Cells were maintained at 37ºC in a humidified atmosphere (90%) containing 5% CO2. Cells were grown in RPMI 1640 medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 100 U/mL penicillin and 100 µg/mL streptomycin.
3.5. Cell Viability
The viability of cultured cells was determined by assaying the reduction of 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) to formazan (29). The study was performed in a 96-well microtiter plate of PC12 cells at a density of 6000 cell/well. After pretreatment with GTAE (7.8 - 62.5 µg/mL) for 24 hours, PC12 cells were exposed to ACR at a final concentration of 10 mmol/L and incubated for 24 hours. Then, cells were treated with MTT solution (final concentration of 0.5 mg/mL in a well) for three hours at 37ºC. The formazan crystals were solublized with dimethyl sulfoxide (DMSO) and the absorbance was measured at 545 nm (630 nm as a reference) in an ELISA reader (Start Fax-2100, UK).
3.6. Experimental Animals
Male Wistar rats weighting 200-250 grams were housed in colony rooms with 12/12 h light/dark cycle at 21 ± 2ºC and had free access to food and water. All animal experiments were performed in accordance with Mashhad University of Medical Sciences, Ethical committee Acts.
3.7. Experimental Design
To induce neurotoxicity in Wistar rats, animals were exposed to ACR at dose of 50 mg/kg in a day via intraperitoneal (IP) injection (3). This daily dose and corresponding route were well characterized with respect to neuropathologic expression and neurological deficits. In this study, rats were divided into six groups randomly (six in each group) and treatment was given as follows:
Control, Normal saline
ACR (50 mg/kg, IP) for 11 days
ACR (50 mg/kg, IP) + GTAE (6.25 mg/kg, IP) for 11 days
ACR (50 mg/kg, IP) + GTAE (12.5 mg/kg, IP) for 11 days
ACR (50 mg/kg, IP) + GTAE (25 mg/kg, IP) for 11 days
ACR (50 mg/kg, IP) + GTAE (50 mg/kg, IP) for 11 days
GTAE (50 mg/kg, IP) for 11 days
3.8. The Behavioral Index (Gait Scores) Examination
After 11 days, the gait scores were examined according to the methods described by LoPachin et al. (30). Rats were placed in a clear plexiglass box and were observed for three minutes. Following observation, a gait score was assigned from 1 to 4, where 1 = normal (unaffected gait); 2 = a slightly affected gait (foot splay, slight hindlimb weakness and spread); 3 = a moderately affected gait (foot splay, moderate hindlimb weakness, moderate limb spread during ambulation); and 4 = a severely affected gait (foot splay, severe hindlimb weakness, dragging hindlimb, inability to rear).
3.9. Statistical Analysis
Results were expressed as mean ± SEM. Statistical analyses were performed with ANOVA followed by Tukey-Kramer test to compare the differences between means. Differences were considered statistically significant when P < 0.05.
4. Results
4.1. Total Polyphenol Content of Green Tea
The total phenolic content was 59.8 mg/g of dried weight.
4.2. Effect of ACR in PC12 Cells
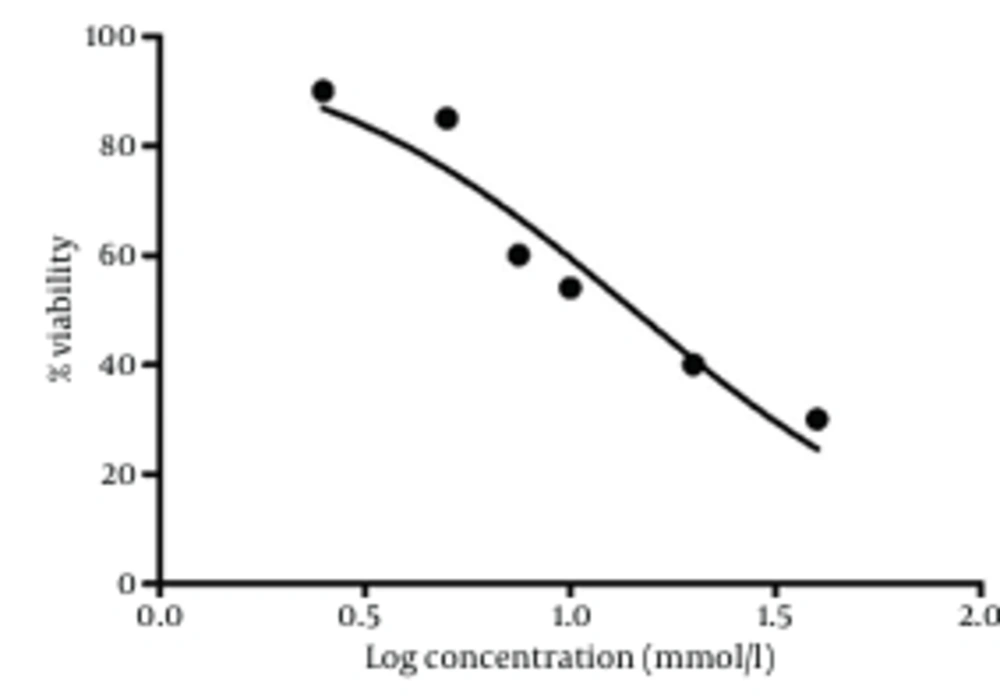
PC12 cells were treated with different concentrations of ACR for 24 hours. Cell viability was measured using MTT test. Treatment of the cells with ACR decreased viability in a dose-dependent manner, as shown in Figure 1. The IC50 (50% inhibitory concentration) value for treatment of PC12 cells with ACR for 24 hours was 10 mmol/L.
4.3. Effect of GTAE on ACR-Induced Cytotoxicity in PC12 Cells
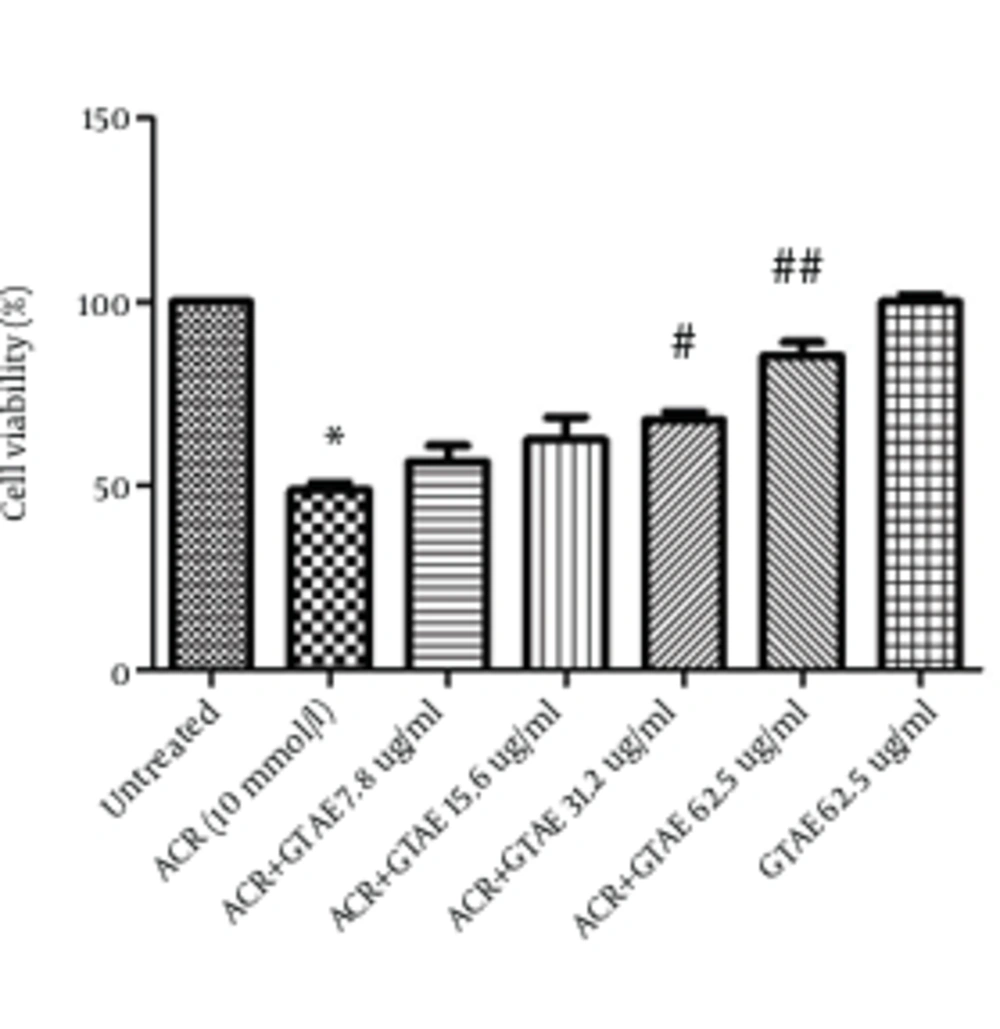
After treating PC12 cells with different concentrations of GTAE (7.8 - 62.5 µg/mL) for 24 hours, the final concentration of 10 mmol/L of ACR was added. After 24 hours of exposure, ACR-induced toxicity was measured using MTT test. The results showed an increase in the cell viability of pretreated cells with GTAE (7.8 - 62.5 µg/mL) compared with ACR group (P < 0.01 and P < 0.001, respectively) which is shown in Figure 2. GTAE alone did not show any cytotoxicity.
4.4. Effect of ACR on the Body Weight in Rats and Protective effect of GTAE
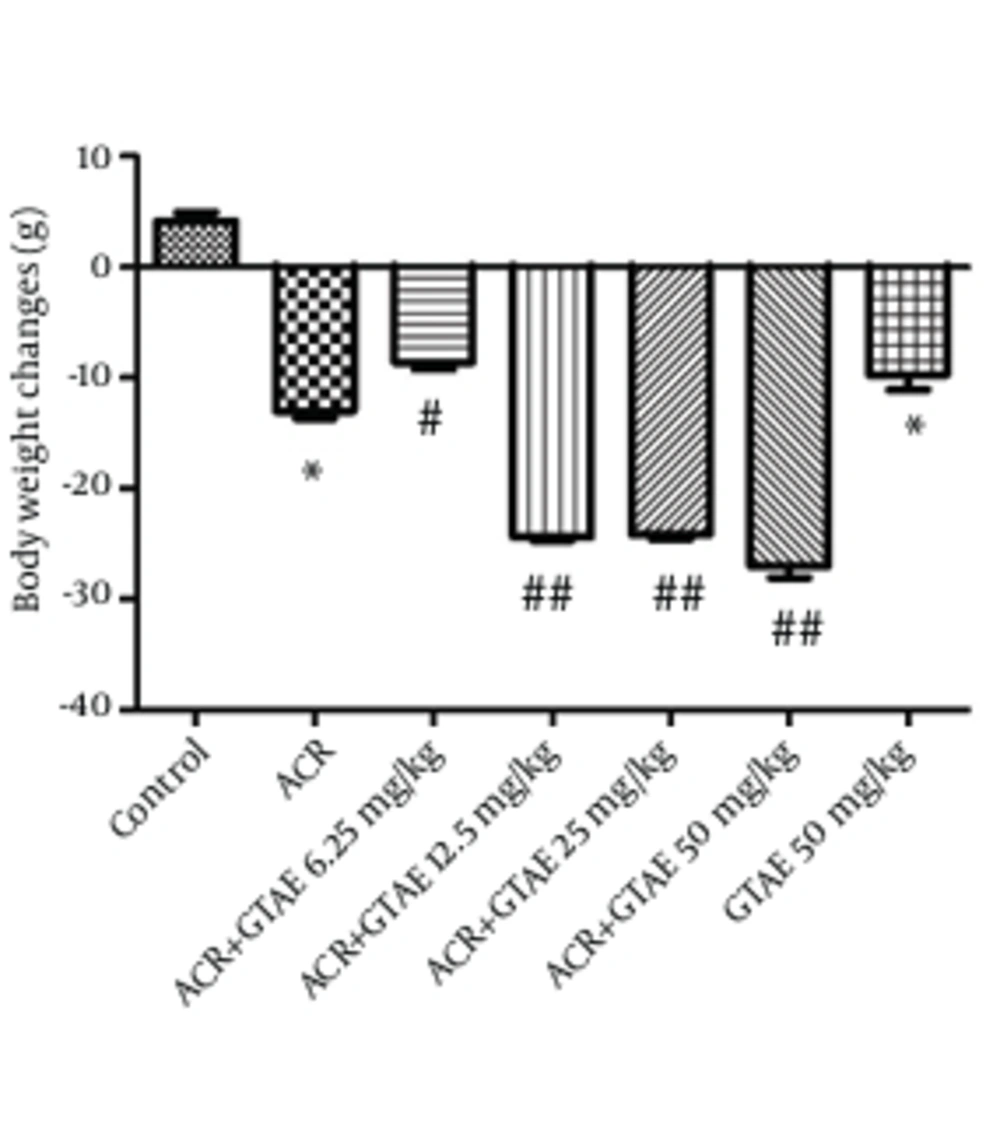
Body weight changes of rats during the treatment are shown in Figure 3. A statistical significant decrease in body weight was observed after 11 days of ACR exposure. Treatment with GTAE decreased body weight after 11 days treatment.
4.5. Effect of ACR on the Behavioral Index (gait Scores) in Rats and Protective Effect of GTAE
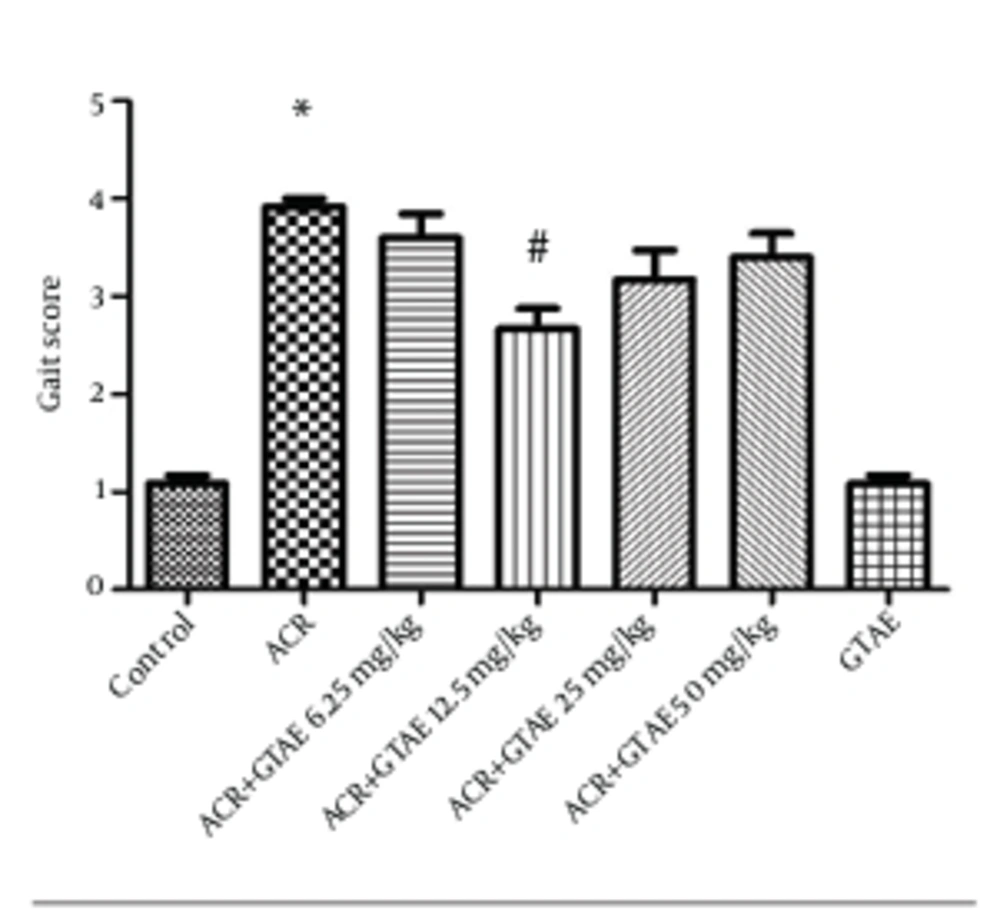
A single administration of ACR (50 mg/kg, IP) for 11 days produced severe gait abnormalities in rats (P < 0.001) (Figure 4). Although coadministration of GTAE (6.25, 12.5, 25 and 50 mg/kg) and ACR decreased gait abnormalities, the effect was only significant in a group which received GTAE at a dose of 12.5 mg/kg plus ACR (P < 0.001).
5. Discussion
In the present study, protective effects of GTAE on ACR induced cytotoxicity in PC12 cells were evaluated as well as ACR induced neurotoxicity in Wistar rats. Our results showed that ACR reduced cell viability in PC12 cells and exposure to GTAE increased the viability of PC12 cells compared with ACR treated cells. Moreover, GTAE pretreated rats showed a greater behavioral index than that of the control group. In the literature, the toxicity of ACR in different in vitro and in vivo models was observed. ACR activated caspase-3 and increased sub-G1 population in SH-SY5Y cells (11, 31). Furthermore, ACR induced apoptosis in PC12 by increasing the bax/bcl2 ratio and activation of caspase 3, which are attributed to the increase of ROS production in PC12 (24). ACR induced apoptosis in neurons and astrocytes in a time and dose-dependent manner and significantly suppressed the proliferation of neural progenitor cells. In addition, apoptotic and necrotic cell death were enhanced in ACR high doses (32).
ACR monomer is a potent neurotoxin and could induce central and peripheral nervous system damages in humans and animals. ACR induces ataxia, skeletal muscle weakness and weight loss in both humans and animal studies (3, 33). Green tea, an infusion from the leaves of C. sinensis Theaceae, is becoming popular as an antioxidant drug (22, 23). The neuroprotective effect of green tea has been documented in different studies. Green tea polyphenols are believed to have the potential as neuropreventive agents for the treatment of neurodegenerative diseases via antioxidant properties (34). Moreover, green tea extract protected ischemia/reperfusion-induced brain cell death by scavenging oxidative damages of macromolecules and inhibited beta-amyloid-induced PC12 cell death (12).
In addition to neuroprotective effects of green tea, several reports showed that green tea could protect other tissues against oxidative damages. It is known that green tea could reduce the oxidation of LDL, which is an important factor in atherosclerosis (35). Additionally, green tea catechins decreased the oxidative stress and hepatic fibrosis (36). In this study, viability of PC12 cells was decreased after 24 hours of exposure to ACR (10 mmol/L). Pretreatment with GTAE (7.8 - 62.5 µg/mL) increased cell viability in a dose-dependent manner. Because of the strong correlation between ROS production and ACR toxicity (13, 37), it is possible that protective effects of GTAE are attributed to the inhibition of ROS generation. Our results showed that treatment of animals with ACR (50 mg/kg, IP) for 11 days decreased body weight and induced severe gait abnormalities (score 4), but treatment of animals with GTAE reduced abnormal gait.
Role of green tea in weight loss has been proved previously. In this study, GTAE 50 mg/kg (the highest dose) decreased mean body weight and mean daily food consumption compared to the control groups, but its effect on mean daily food consumption was not significant (data was not shown). Therefore, it could be suggested that other mechanisms including inhibition of the enzymes catechol-o-methyltransferase, acetyl-CoA carboxylase and fatty acid synthase as well as reducing fat absorption via the gut, may be involved in green tea weight reducing effect. According to our results, ACR caused a significant reduction in body weight after 11 days of treatment. Besides, GTAE led to body weight loss, which could be due to antiobesity effect of green tea (17). Our data also indicated that increasing the dose of GTAE (12.5 mg/kg to 50 mg/kg) decreased green tea neuroprotective effect and the effect of GTAE (12.5 mg/kg) on ACR induced neurotoxicity was more than other selected doses. This may be related in part to body weight loss induced by green tea in higher doses.
As green tea is a source of potent antioxidants, it could be suggested that protective effect of GTAE against ACR toxicity, both in vitro and in vivo experiments, may be related to antioxidant effects of green tea and its constituents.