1. Background
Oral diseases continue to be a major health problem worldwide. Various bacteria and fungi are found to be the possible pathogens responsible for the oral diseases (1). Dental caries and periodontal diseases are among the most important global oral health problems. Streptococcus mutans is one of the main opportunistic pathogens for dental caries (2), which plays a central role in demineralization of the tooth enamel (3). There are several virulence factors that make these bacteria cariogenic, including adhesion, acidogenicity and acid tolerance (4). In addition, other microflora like Escherichia coli and Candida albicans are also associated with active carious lesions. Candidaalbicans is the most common yeast isolated from the oral cavity and a common cause of oral thrush, endocarditis, septicemia, vaginitis and infection of skin, nails and lungs. It is by far the most common of the fungal species most commonly isolated from infected root canals, showing resistance to intercanal medication (5). Poor oral hygiene is one of the reasons for accumulation of these microbes and their harmful activities (3).
According to Carranza, plaque control is the most effective way for preventing the development of the oral diseases. Most of the oral microbial diseases can be prevented by effectively removing plaque deposition from the teeth surfaces by the use of the mechanical aids, such as a toothbrush (6). Although people try to maintain their oral hygiene, many of them cannot remove plaque, and therefore mouth rinses are used to complete the process of plaque removal (7). Although mouth rinses have been used for centuries for medicinal and cosmetic purposes, but it is only in recent years that the rationale behind the use of chemical ingredients has been subject to scientific research and clinical trials (8, 9). The most common commercially used mouth rinses are chlorhexidine (CHX), essential oils, triclosan and sodium fluoride (S-Flo).
The chemical plaque control agents such as CHX can be useful adjuncts for plaque control. Nevertheless, they can only be used as supplement and not as substitute to the mechanical plaque control. They have been found to be effective in the control of plaque and gingivitis, as demonstrated in the various studies (9). Topical fluoride promotes remineralization and inhibits demineralization of enamel during caries process. Another effect of fluoride includes the inhibition of glycolysis, coupled with a reduction in the production of extracellular polysaccharide (10, 11).
There are multiple natural compounds like xylitol, green tea, mint and propolis that had been tested as mouth rinses. Propolis, sometimes called as bee glue, is a natural resinous substance collected by honey bees (Apis mellifera L.) from plant buds and bark exudates. Propolis is a very complex mixture and its chemical constituents vary according to its source. Bioflavanoids are the key contributors to the antimicrobial properties of propolis (12).
The potential of propolis, as a natural antibiotic, has long attracted the scientific interest (12). Propolis has been found effective in inhibiting several species of microbes, such as bacteria, viruses and fungi, which explains its antimicrobial action (13, 14).
2. Objectives
The present in vitro study was conducted to assess the antimicrobial efficacy of MR prepared from Brazilian propolis, as compared to S-Flo MR and CHX MR against common oral pathogens like S. mutans, C. albicans and E.coli.
3. Materials and Methods
The efficacy of three MRs and negative control group (0.89% normal saline) were evaluated against S. mutans, C. albicans and E. coli. The four solutions were grouped as follows:
MR1: Chlorhexidine gluconate solution 0.2% w/v (Hexidine mouthrinse, ICPA Health Products, Ankleshwar, India) - CHX
MR2: Sodium-fluoride mouthrinse U.S.P 0.2% w/v (Aggarwal Drugs Pvt. Ltd., Haridwar, Uttarakhand) - S-Flo
MR3: Propolis and neutral alcohol of cereal (Natucentropropolis, Bambui, Brazil).
MR4: The 0.89% normal saline served as the negative control group (prepared and sterilized in lab).
This study was conducted at the Department of Microbiology, Sudha Rustagi College of Dental Sciences and Research, Faridabad (Haryana), India.
3.1. Preparation of Culture Media
Preparation of culture media was done in conformity with the manufacture’s specifications (Himedia Laboratories, Mumbai, India). Sterilization of the culture media was done by autoclaving at 15lbs psi (per square inch) for 15 minutes. These media were poured in disposable sterilized plastic petri plates to obtain a matrix thickness of 4 mm.
3.2. Bacterial Cultures
Freeze dried cultures of S. mutans (MTCC 890), E. coli (MTCC 44) and C. albicans (MTCC 1637) were obtained from Microbial Type Culture Collection (MTCC) and Gene Bank, Institute of Microbial Technology, Chandigarh, India.
3.2.1. Antimicrobial Testing
The antimicrobial activities of the three MRs were tested using agar well diffusion method.
The cultures, which were kept in the refrigerator at 4°C, were reconstituted by adding 500 µL of brain-heart infusion (BHI) broth and tubes were incubated at 37°C, for 24 hours. These cultures, viz: S. mutans, E. coli, C. albicans, were plated on BHI agar, nutrient agar and sabouraud dextrose agar, respectively, and incubated at 37°C for 24 hours.
The purity of culture was confirmed by Gram staining before use. The colony growth of the respective cultures was passed in 4 mL of sterile normal saline, in a tube. The tubes were shaken to have a visible turbidity. The surface of culture media plates was dried by keeping the plates open in the incubator for 4 hours.
Normal saline bacterial suspension was flooded on to the surface of the dried plates. Extra culture inoculum was decanted off from the surface of the medium, back into the culture tubes. The plates were incubated at 37°C, for about 45 minutes, for the liquid culture to absorb in the matrix. Using a borer, which had been flamed red hot and subsequently cooled, wells having a diameter of 6 mm and depth of 4 mm were made in the plates. Four wells were punched in each petri plate.
Each experimental and control agent was placed over four wells in each of the three prepared agar bases and 100 μL of each agent/combination was pipetted into the wells. The plates were kept for 1 hour at room temperature for diffusion of agent through the medium. Afterwards, the plates were incubated at 37°C for 24 hours, with lid upwards. This work was carried through in laminar air flow station.
Following this incubation, inhibition zone diameters (in mm) were measured along the most uniform diameter, using a transparent plastic scale.
Mean inhibition zone diameters were recorded and results were subjected to one-way analysis of variance (ANOVA), test using the SPSS version 20 statistical software (SPSS Inc., Chicago, IL, USA). Inter-group comparison of mean values was done using the post-hoc Tukey’s honest significant difference (HSD) test (P < 0.05)
4. Results
The results for the antimicrobial efficacy of various MRs against three different organisms were as presented in Table 1.
| Compounds | Microorganisms | P Value b | Post Hoc Test | ||
|---|---|---|---|---|---|
| Streptococcus mutans | Candida albicans | Escherichia coli | |||
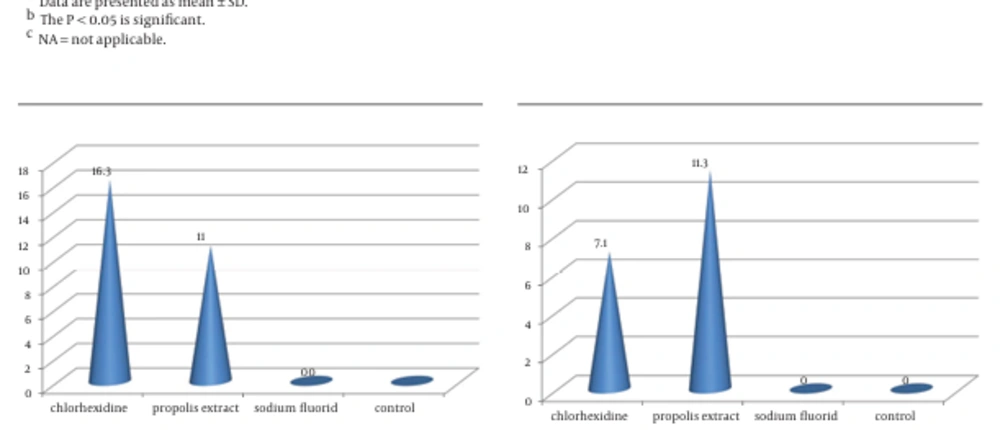
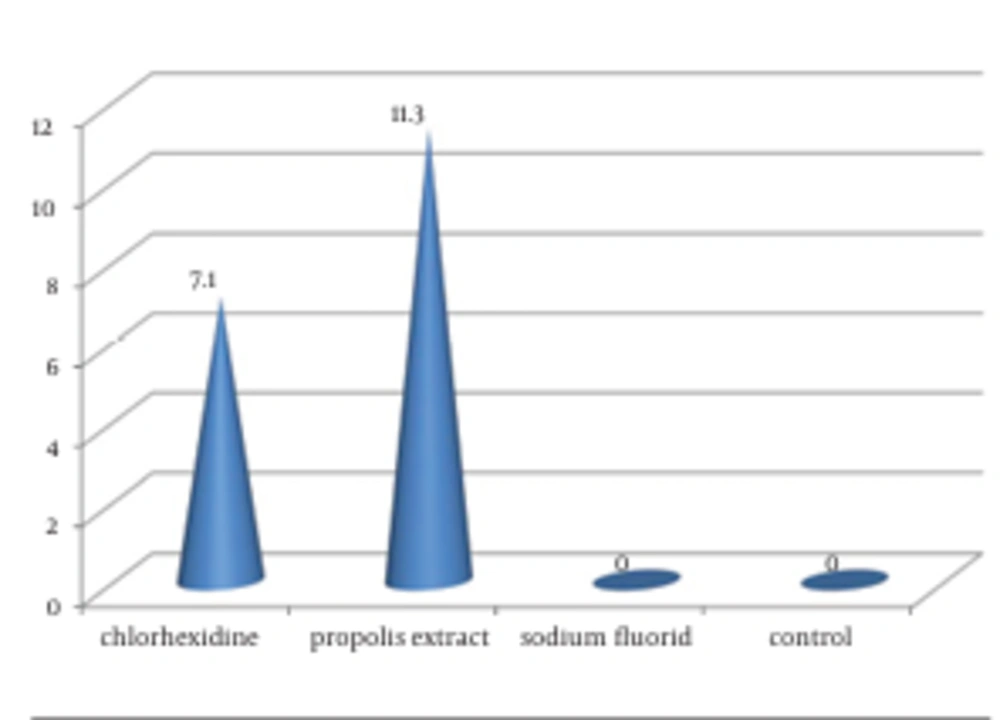
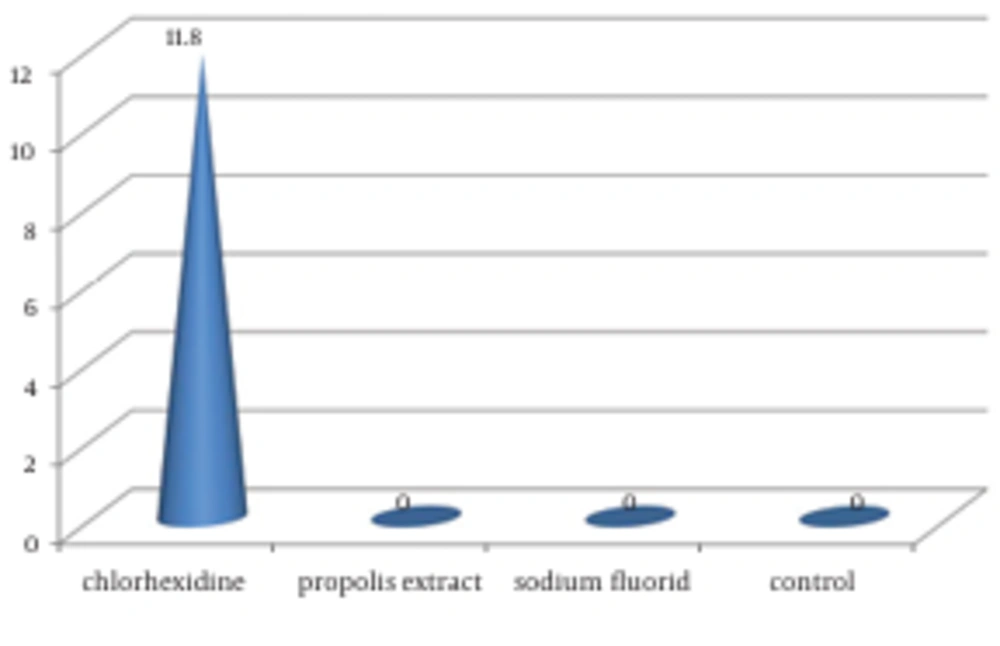
| Chlorhexidine | 16.30 ± 0.48 | 7.10 ±1.10 | 11.80 ± 1.03 | < 0.001 | C. albicans < E. coli < S. mutans |
| Propolis extract | 11.00 ± 0.67 | 11.30 ± 1.16 | 0 | < 0.001 | E. coli < S. mutans < C. albicans |
| Sodium fluoride | 0 | 0 | 0 | NA c | NA |
| Normal saline | 0 | 0 | 0 | NA | NA |
Comparison of Zone of Inhibition Produced by Test Solutions Against Three Microorganisms According to the Microorganism a
4.1. Streptococcus mutans
The CHX followed by propolis had a significantly larger zone of inhibition than S-Flo and the control group (Figure 1).
4.2. Candida albicans
Propolis extract MR (11.30 ± 1.160) and CHX (7.10 ± 1.101) showed significantly (P < 0.05) larger zones of inhibition in comparison to S-Flo (0) and the control group (0), whereas propolis had a significantly (P < 0.05) greater zone of inhibition than CHX (Figure 2).
4.3. Escherichia coli
Only CHX MR showed zone of inhibition (11.80 ± 1.033) against E. coli. The results for antimicrobial efficacy of individual MR against the three organisms are depicted in Table 2 and Figure 3.
| Microorganisms | Compounds | P Value b | Post Hoc Test | |||
|---|---|---|---|---|---|---|
| Chlorhexidine | Propolis Extract | Sodium Fluoride | Control | |||
| Streptococcus mutans | 16.30 ± 0.48 | 11.00 ± 0.67 | 0.00 | 0.00 | 0.000 (< 0.05) | Chlorhexidine > Propolis Extract > Sodium Fluoride, and Control |
| Candida albicans | 7.10 ± 1.10 | 11.30 ± 1.16 | 0.00 | 0.00 | 0.000 (< 0.05) | Propolis Extract > Chlorhexidine > Sodium Fluoride = Control |
| Escherichia coli | 11.03 ± 1.03 | 0.00 | 0.00 | 0.00 | 0.000 (< 0.05) | Chlorhexidine > Propolis Extract, Sodium Fluoride, and Control |
Comparison of the Zone of Inhibition Produced by Four Test Solutions Against Microorganism According to the Mouthwash a
4.4. Chlorhexidine
Chlorhexidine MR showed a significantly (P < 0.05) higher zone of inhibition against S.mutans (16.30 ± 0.48) in comparison to E. coli (11.80 ± 1.03) and C. albicans (7.10 ± 1.10). Mean zone of inhibition produced by CHX MR was significantly higher against E. coli in comparison to C. albicans.
4.5. Propolis Extract
Propolis extract MR showed a significantly (P < 0.05) larger zone of inhibition against C. albicans (11.30 ± 1.16) in comparison to S. mutans (11.0 ± 0.67) and E. coli. Mean zone of inhibition produced by propolis extract was significantly higher against S. mutans, in comparison to E. coli.
4.6. Sodium Fluoride
Sodium fluoride MR did not show any zone of inhibition against all three test organisms, demonstrating no antimicrobial activity.
5. Discussion
Dental plaque harbors the microorganisms and is primarily responsible for the occurrence of various dental diseases (2). The plaque formation is a multistep process which consists of a sequential colonization of the bacteria on the tooth surface, beginning from the attachment of the first species onto the tooth surface, followed by the evolution into the highly evolved species (2, 3).
Most of the oral diseases are a result of the activity of various microorganisms present in the plaque, which need to be kept in check. Therefore, agents which can inhibit these microorganisms have represented an area of interest in research (15). These substances act by either killing the microorganisms, or by disrupting their cell walls, or by inhibiting their enzymatic activity. They also prevent bacterial aggregation, slow multiplication and release of endotoxins (16). Several clinical studies have demonstrated the inhibitory effects of antimicrobial MRs on oral bacteria (17).
In the present study, CHX MR was found to be significantly (P < 0.001) more efficacious against S. mutans and E. coli than all other tested MR. Similar results have been reported by Malhotra et al. (18), which showed that Hexidine (0.12% chlorhexidine gluconate) MR had the best antimicrobial efficacy against all the tested microorganisms, with laboratory-manufactured propolis MR showing an equivalent efficacy only against S. mutans. According to the study conducted by Aneja et al. (19), Hexidine MR emerged as the most effective MR, with a maximum mean diameter of the inhibition zone against S. aureus, followed by S. mutans, S. cerevisiae, and minimum against C. albicans. Similar results have been reported in the studies conducted by Nakamoto et al. (20), which showed that CHX gluconate has an inhibitory effect on the growth of C. albicans.
Chlorhexidine is a cationic bisbiguanide with antimicrobial properties, which depends on its concentration, acting as bacteriostatic at low concentrations and bactericidal at high concentrations. It has the capacity to inhibit all known microbes in the oral cavity, therefore contributing to its broad-spectrum activity and being considered the gold-standard for plaque control.
The efficacy of Brazilian propolis used in the present study was found to be more substantial against S. mutans and C. albicans, as compared to E. coli. Although the efficacy of the propolis extract was lower than that of CHX MR for S. mutans, the efficacy of propolis extract was superior to that of CHX for C. albicans. Similar results have also been demonstrated by Bruschi et al. (21) and Ugur and Arslan (22). According to the study conducted by Ugur and Arslan, the most sensitive microorganism to propolis were Shigella sonnei, in the gram-negative group, S. mutans, in the gram-positive group, while the least sensitive microorganism was C. albicans (22). However, the study conducted by Malhotra et al. (18) showed that laboratory manufactured propolis MR has an equivalent efficacy against S. mutans when compared to CHX MR.
Duailibe et al. (23) observed that propolis extract possesses in vivo antimicrobial activity against S. mutans. This can be attributed to the following properties: antibacterial activity due to components like the flavonoids, which are considered to be the principal components for its biological activities (24) and anticariogenic effects (25). The different varieties of flavonoids have been reported in different types of propolis, which contribute to its cariostatic activity. The variation in cariostatic activity is due to the varied composition of the flavonoids as a result of the different sources from which propolis is obtained (25-27).
Propolis, due to its availability and no reported side-effects up to present date, can be a useful option. The effectiveness of propolis is different according to the region from which it is obtained, due to a change in the chemical composition from area to area (24). It has a varied effect on the bacteria, being more effective against gram-positive bacteria (28-30). Although the antimicrobial properties of propolis have been evaluated by several investigations, it is difficult to compare the results of different studies due to the different methods used (31). However, the results of the study conducted by Elbaz et al. (4) reveal that the New Zealand propolis lozenges had a potent antimicrobial activity.
The efficacy of the S-Flo MR was lower than CHX and propolis, against all the tested microorganisms. Most of the in vivo studies have shown a cariostatic effect of fluoride gels or MR at 1% or 2% fluoride concentration (32). Consequently, the observed lower efficacy of S-Flo could also be attributed to the lower percentage of fluoride (0.2% of NaF) used in this study.
Meurman (32) studied the ultrastructure, growth, and adherence of S. mutans ATCC 27351 to hydroxyapatite, after treating bacterial suspensions for 1 hour with 0.1% CHX gluconate, 0.1% S-Flo, and a combination of the two. The fluoride treated specimens appeared the same as the controls, while the ultrastructure was mostly normal. Treatment with fluoride alone did not cause alterations in the ultrastructure or reduction in adsorption of S. mutans. Nevertheless, the study conducted by Gamal El-Din et al. (11) showed the presence of a statistically significant difference between the effect of both types (S-Flo and Propolis) on mean S. mutans count in oral cavity of girls and also, a statistically significant difference between their effect on the change in S. mutans count in both sexes during the follow-up period.
This testing method functioned only as a screening method and does not prove similar efficacy when used as a MR. There is a definite reduction in the level of bacteria and other pathogenic microorganisms in saliva (31, 33, 34) and mucosa (17, 35), which has been warranted by multiple studies assessing the efficacy of the antimicrobial MR for the prevention of the oral disease. However, antimicrobial efficacy was checked in vitro, and therefore it cannot be assumed that the results of antimicrobial efficacy could be proportional or transferable to the oral cavity and translated into clinical effectiveness. Consequently, from the overall results obtained, it is evident that various MRs listing CHX and propolis extract as the active ingredient demonstrate different antimicrobial activities.