1. Background
The antimicrobial activity of Punica granatum Linn has been widely investigated (1, 2). The findings of several studies suggest that the phytotherapeutic use of pomegranate can be a viable option in controlling different microbial species. The greatest components of the Punica granatum L. fruit extract are polyphenolics and tannin (3).
Punica granatum L. (pomegranate) was originally grown in Iran, Afghanistan, India and China while, it is now cultivated in several other regions such as Mediterranean, South Africa, America and Mexico (4). In Iranian traditional medicine pomegranate has been used as a vermifugal, and for recovery of wounds, relieving diarrhea and gastric inflammation (5). The pharmacological properties of various parts of pomegranate have been studied extensively (1, 6, 7). The findings of several studies, suggest that the phytotherapeutic use of this plant might be a viable option in controlling different microbial species. Pomegranate is a rich source of bioactive compounds, specifically polyphenolic compounds. Punicalagin isolated from the fruit peel of pomegranate was reported to have antimicrobial activity against Candida albicans (2).
Invasive infections caused by Candida spp. are associated with high mortality and a major healthcare problems in modern medicine (8). Candida species are ranked as the third most common nosocomial bloodstream pathogen isolates in hospitals of western countries (9, 10), while they are associated with the highest mortality among bloodstream infections. However, resistance to antibiotic therapy has been a significant public health problem in the last three decades (11). Shokohi et al. (12) reported that 17.4% of Candida isolates of oropharyngeal lesions of patients with cancer were resistant to antifungal agents. Prevention and control of these infections will require new antifungal agents, prudent use of existing agents, new vaccines, and enhanced public health efforts to reduce morbidity, mortality, and health-care costs (13).
Therapy for Candida infections has become a challenge. Treatment is difficult due to the eukaryotic nature of fungal cells, which are similar to host cells. A few antifungal agents are in clinical use, and therefore therapy is limited by drug-safety considerations and their narrow spectrum of activity, efficacy and resistance (14). Candida species have become resistant to antifungal agents, where sometimes more than 50% of patients are infected with a resistant organism. The clinical consequences of antifungal resistance can be seen in treatment failures, and changes in the prevalence of infections due to Candida species (15).
The developments and increase of fungal infections (16) has necessitated the development of novel treatment, efficacious, safer and more potent agents, and broader spectrum antifungal agents. On the other hand, plants have been used since antiquity to treat health disorders and to prevent diseases (17). Medicinal plants are well-known natural sources for the treatment of various diseases for thousands of years (18). Identifying the antifungal potential of many traditional plants is growing, mainly due to the fact that a lot of synthetic drugs are potentially toxic and are not free of side effects in patients.
There is a growing interest in using tannins as antimicrobial agents in caries prevention and fungal counts in periodontal disease and Candida-associated denture stomatitis. (19, 20).
Many plant species containing hydrolysable tannins have been used for the treatment of diseases, especially in Asia (21). Iran is amongst the most biologically diverse countries in the world with over 1000 cultivars of Punica granatum (22) originating from Iran, the Middle East, India, China, Mexico, Mediterranean region and the USA.
Pomegranate (P. granatum) is a rich source of hydrolysable tannins (punicalin, pedunculagin, punicalagin, gallic and ellagic acid) that have antifungal activities, and anthocyanins along with phenolic compounds, which have strong antioxidant activities. Although pomegranate’s wide-ranging therapeutic benefits may be attributable to several mechanisms, most research have focused on its antioxidant, anticarcinogenic and anti-inflammatory properties (20). Its antifungal mechanism may involve permeabilization of fungal cell membrane, induction of reactive oxygen species and triggering of apoptosis (23). From this perspective, pomegranate (P. granatum) may be interesting for systematic modern scientific explorations in achievement of novel antifungal agents.
2. Objectives
The primary objective of this study was to investigate the potential efficacy of pomegranate peel and pulp extracts to provide opportunities for a safe and an efficient antifungal drug, and to evaluate in vitro antifungal activities of various extracts of eight Persian pomegranates peel and pulp cultivars against five standard strains of Candida (C. albicans, C. parapsilosis, C. tropicalis, C. glabrata and C. krusei).
3. Materials and Methods
3.1. Plant Material
The eight cultivars of Persian Punica granatum L. (pomegranate) peel and pulp (sour summer, sour white peel, sweet white peel, sour malas, sweet malas, seedless white peel, Agha Mohammad Ali and black peel) used in this study were purchased from mature fruits grown in the collection of the Agricultural Research Center of Saveh (Iran), during September 2013.
3.2. Extract Preparation
A total of eight pomegranate cultivars (pulp and peel) were collected and washed. The pomegranates were manually peeled, dried and powdered, using a mixer grinder. The extract was prepared by extraction of the pomegranate powders (120 grams) in a soxhlet extractor using 80% methanol for 10 days. After extraction the solvent was removed, by means of a rotary evaporator, and its yield was calculated as 69 ± 0.9%
3.3. Strains and Growth Conditions
The following Candida strains were used in the present microbiological assays: C. albicans (ATCC 10231) C. parapsilosis (ATCC 22019), C. tropicalis (ATCC 750), C. glabrata (PTCC 5297) and C. krusei (PTCC 5295). The Candida strains were cultured in Sabouraud-dextrose broth medium (Difco) and incubated under aerobic conditions at 35°C for 48 hours. All strains were purchased from the Iranian Research Organization for Science and Technology (IROST).
3.4. Micro Dilution Method
The minimal inhibitory concentration (MIC) of the crude extract and pure compound against the above-mentioned strains were determined according to standard guidelines of M27-A2 (antifungal susceptibility testing) broth micro dilution reference procedure of the Clinical and Laboratory Standards Institute (CLSI) (2002) (24). This standard in vitro susceptibility testing of yeasts (M27-A2), provides guidelines that suggest the use of RPMI-1640 as the test medium, inoculums prepared spectrophotometrically with a final test concentration of 0.5 to 2.5 × 103 CFU/mL, incubation temperature of 35°C for 48 hours, and the criteria for determining the endpoint or MIC.
The crude extract was dissolved in distilled water (100 μg/100 mL). Serial two-fold dilutions of the crude peel and pulp extract were done in a microdilution plate (96 wells) containing 100 μL of sterile RPMI-1640. The inoculums were then added to each well. The inoculums were adjusted for each microorganism to yield a cell concentration of 2 × 103 CFU/mL. Positive and negative controls were also used.
The sealed microplates were incubated at 35°C for 48 hours. The MIC was defined as the lowest concentration, which resulted in inhibition of growth. Since the pomegranate peel had color pigments, the results could not be obtained by the ELISA method due to the instrument’s fault. Therefore, minimal fungistatic concentrations were determined by sub-culturing 10 μL of the culture from each well and from the positive and negative control on sabouraud-dextrose agar (Difco). Fluconazole 2% (Sigma-Aldrich, USA) was used as the positive control. All testing was done in duplicates. The lowest concentration of the compound that completely inhibited macroscopic growth was determined and MICs were reported as described previously (25, 26).
3.5. Statistical Analysis
Comparative analysis of antifungal activity of the eight pomegranate cultivars against five Candida species was assessed by the Kruskal-Wallis Test. A P value of < 0.05 was considered statistically significant. Descriptive statistics were presented as medians, means and standard deviations. The Kolmogorov-Smirnov test was used to assess the normal distribution of variables. Three replicates of each sample were used for statistical analysis and these statistical comparisons were performed using the SPSS software version 16.
4. Results
Five Candida species were tested for their sensitivity to eight cultivars of the Persian Punica granatum (pomegranate) peel and pulp extracts. The yield of pomegranate peel and pulp extract was calculated as 69 ± 0.9%. The antifungal potency was determined by the minimum inhibitory concentration (MIC) method.
This study demonstrated that flavonoid-rich pomegranate peel extract could inhibit growth of Candida species. All five Candida strains (C. albicans, C. tropicalis, C. parapsilosis, C. krusei, and C. glabrata) were sensitive to the peel extract of Punica granatum (pomegranate) in vitro.
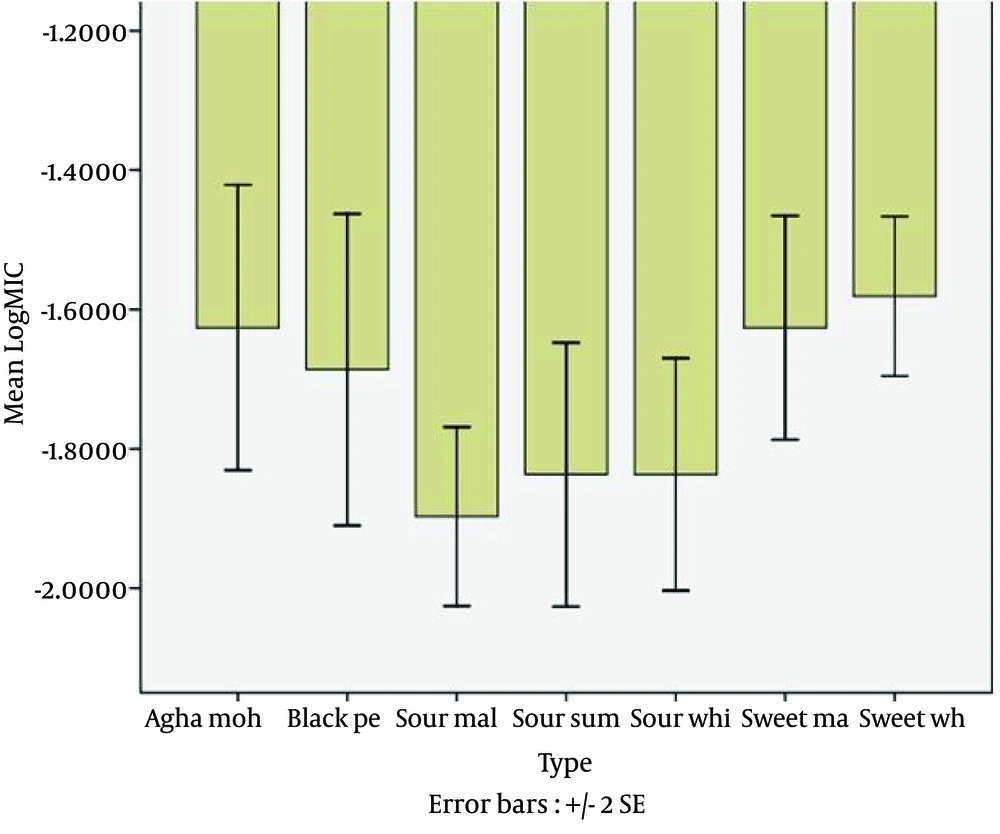
The antifungal activity of eight cultivars of Pomegranate peel extracts against C. albicans (ATCC 10231), C. parapsilosis (ATCC 22019), C. tropicalis (ATCC 750), C. glabrata (PTCC 5297) and C. krusei (PTCC 5295) are shown in Figure 1.
Statistical analysis showed that the greatest antifungal activity among the eight different cultivars against Candida species was observed by sour malas, sour white peel and sour summer peel extracts, respectively (Figure 1). In this study, the sour malas pomegranate (Punica granatum L.) peel extract had greater efficiency than the other cultivars. The sour malas extract demonstrated the best antifungal activity against C. albicans (ATCC 10231), C. parapsilosis (ATCC 22019), C. tropicalis (ATCC 750), C. glabrata (PTCC 5297) and C. krusei (PTCC 5295) with MIC values of 125μg/mL, 125μg/mL, 62.5 μg/mL, 62.5 μg/mL and 62.5 μg/mL, respectively. Table 1 demonstrates the sure malas peel extract was more effective than the other cultivars against Candida spp.
| Cultivar | Candida albicans | Candida parapsilosis | Candida tropicalis | Candida krusei | Candida glabrata |
|---|---|---|---|---|---|
| Sour summer, μg/mL | 125 | 125 | 250 | 62.5 | 62.5 |
| Sour white peel, μg/mL | 125 | 250 | 62.5 | 125 | 62.5 |
| Sweet white peel, μg/mL | 250 | 250 | 62.5 | 125 | 125 |
| Sour malas, μg/mL | 125 | 125 | 62.5 | 62.5 | 62.5 |
| Sweet malas, μg/mL | 250 | 250 | 62.5 | 125 | 125 |
| White peel seedless, μg/mL | 250 | 62.5 | 250 | 125 | 62.5 |
| Agha Mohammad Ali, μg/mL | 250 | 250 | 250 | 62.5 | 125 |
| Black peel, μg/mL | 250 | 62.5 | 125 | 125 | 62.5 |
In addition, the minimum inhibitory concentration of Punica granatum L. peel extracts was 250 μg/mL against Candida spp. which was comparable to 2% fluconazole. The antifungal activity of pulp extracts against Candida species was insignificant.
However, the MIC value of the black peel cultivar pulp extract against C. albicans (ATCC 10231), C. parapsilosis (ATCC 22019), C. tropicalis (ATCC 750), C. glabrata (PTCC 5297) and C. krusei (PTCC 5295) was more than 250 μg/mL, which was the highest test concentration. The other cultivars of pomegranate pulp extracts did not inhibit Candida species growth by micro dilution methods. In fact seven cultivars of pomegranate pulp extracts confirmed the negative results.
5. Discussion
This study evaluated the anti candida activity of Persian pomegranate commonly consumed in Iran. Resistance of microorganisms to antimicrobial agents is becoming a growing concern worldwide. Herbal drugs are a credible part of the treatment of mycosis alongside pharmaceutical antifungal drugs. Several studies have demonstrated the antifungal potential of the active ingredients of pomegranate extracts (2, 27, 28).
In the present study we evaluated the antifungal activity of peel and pulp of eight different pomegranate cultivars against five Candida strains. We found that peel extracts were the most significant part of the fruit with highest antifungal activity. The results showed that, three peel cultivars including sour malas, sour white peel and sour summer had the most antifungal content among eight different cultivars against Candida species.
In a study by Shams Ardekani et al. sour summer peel and pulp had the most total phenolic compounds (35.92 ± 0.84 and 21.03 ± 1.51, respectively), when compared to other cultivars (29). Also they revealed that the Sour white peel had the most flavonoid/phenolic compounds when compared to the nine other cultivars. Also a study by Sadeghi et al. (30) revealed that sour white peel had the highest potent antioxidant activity among different pomegranate cultivars. This may be attributed to the greater potential anti-candidal activity against five varieties of Candida species in sour pomegranate peel extract in the present study. A variety of cultures and traditions in developing and developed countries recommend pomegranate peel to treat common health problems (31). Pomegranate has strong antioxidant properties and this has been attributed to its high polyphenolic content. The most important phenolic compounds and organic acids in pomegranate peels are flavonoids (anthocyanins, catechins and other complex flavonoids) and hydrolysable tannins (punicalin, pedunculagin, punicalagin, gallic and ellagic acid). Ellagic acid derivatives of tannic acid, and tannins such as gallic acid, and gallagic-derived tannins are considered as punicalagin (32). There is a growing interest in using tannins as antimicrobial and antifungal agents (20). The action of tannins against bacteria and yeasts can be the determinant of such antibacterial and antifungal activity, with dependence on their molecular structure, toxicity, astringent properties or other mechanisms (7).
The samples of sour pomegranate peel extracts, which were more effective against Candida species, had a high concentration of punicalagin compounds, in addition to other tannins, such as pedunculagin and tellimagrandin. The pomegranate peel sample also included the tannin gallagyldilacton (27).
The ellagitannin, punicalagin, is thought to be the fraction responsible for pomegranate’s antibacterial activity (33). Although pomegranate’s wide-ranging therapeutic benefits may be attributable to several mechanisms. Presence of phytoestrogenic flavonoids (luteolin, kaempferol and quercetin) have been reported in pomegranate peels by van Elswijk et al. (34). Pomegranate is an important source of bioactive compounds. The largest components of the Punica granatum L. extract are tannin and polyphenolics (3). The action of tannins against Candida can be related to their molecular structure and toxicity, astringent properties or other mechanisms. The effect of tannins on microbial metabolism can be measured by their action on membranes (8). They can transmit the cell wall, combined to several polysaccharides and proteins, and bind to its surface. This adhesion can also help determine the minimum inhibitory concentrations for yeasts and bacteria (5).
Another study reported high antifungal activity by punicalagin against C. albicans and C. parapsilosis, indicating that this substance is a significant antifungal agent; however, the mechanism of action was not elucidated (7).
Different mechanisms explain the action of tannins against the Candida genus. Antifungal activity can be established by inhibition of extracellular microbial enzymes, deprivation of substrates and metal ions required for microbial growth, and direct action on microbial metabolism through inhibition of oxidative phosphorylation (35).
These bioactive compounds could be responsible for changes in cell morphology, inhibition of growth, production of viscous material and rupturing of the cells (35). In addition, tannins are able to form complexes with other molecules, including macromolecules such as proteins and polysaccharides (19). The findings of several studies, including some related to inhibition of adherence have suggested that oral bacteria and C. albicans are sensitive to the extract of P. granatum (6, 34). Furthermore, punicalagin isolated from the peel of pomegranate was reported to have antifungal activity against Candida albicans (36). Interestingly, the antifungal activity of sour peel extracts was found to be more than the sweet peel extracts of P. granatum L. In the study by Naziri et al. (37) it was shown that MICs for sweet pomegranate peel extract were generally lower than those for sour pomegranate peel extract (P < 0.05).
As the MIC value for sweet pomegranate peel extract and MBC (minimum biocidal concentration) value for sour pomegranate peel were found to be relatively low (P < 0.05), it can be concluded that the extract of sour pomegranate peel may exert a high bacteriostatic effect. On the other hand the extract of sweet pomegranate peel may have a lower bacteriostatic effect. These differences may be due to variations between the amount of antibacterial substances such as tannins and phenolic substances, in sour and sweet pomegranate peels. In a study by Fazeli et al. (38), pomegranate sour juice showed the highest activity against Pseudomonas aeruginosa with wider zones of growth inhibition than pomegranate sweet juice. In a study by Shams Ardekani et al. sour summer had a high potential for extraction and purification of phenolic and flavonoid compounds. Also, they showed that sour summer peel extract has the most Fe2 +, vitamin C and E when compared to the other nine pomegranate cultivars of Saveh (29).
The phenolic compounds extracted from pomegranate have the ability to inhibit gram negative and gram-positive bacteria (39). Phenolic substituted flavonoids have also been shown to possess more effective antifungal activity against Candida species when compared to other flavonoids (40). Therefore, it can be concluded that the extract of sour pomegranate peel may exert a high fungistatic effect, although we did not find any studies on sour and sweet pomegranate fungistatic effects in the literature.
Reduction of C. albicans adherence on cover glasses and morphological alterations were caused by treatment with punicalagin (18). Various bioactivities of pomegranate are attributed to the presence of tannins, in particular, ellagitannin, which is the major component of pomegranate extracts (41). The present study demonstrated that the flavonoid-rich pomegranate peel extract could have anti-candidal activity against five varieties of Candida species. The mechanisms of action of these compounds are likely to be an attractive subject of research.
The results of the susceptibility tests with pomegranate peel extract in this study, could give prescribers more confidence when choosing an alternative type of antifungal agent. A more integrated approach is needed to use pomegranate peel for the treatment of candidiasis, especially for immunocompromised patients that have received other treatments for their diseases. Similarly, in vivo trials are needed to assess the potential of this fruit in treating fatal in vivo fungal infections. Clinical efficacy may be improved by better understanding of their mechanisms of action on fungal infections. With consideration of the resistance of fungi to common antifungal agents, this paper proposed the possibility of using bioactives from pomegranate peel to solve the increasing problem of fungal resistance. The obtained data opens new perspectives for future research in continuation of this study, where information such as definition of the site of action of the sour malas cultivar Persian pomegranate peel could contribute to an alternative therapy against candidiasis.
Therefore, the antifungal characteristics of natural pomegranate compounds can represent an interesting feature for their application in oral candidiasis. The results obtained in this study clearly demonstrate the broad-spectrum antifungal activity of pomegranate (Punica granatum L.) peel extract against Candida strains. Pomegranate peel exhibits a high antifungal potential. It has gained wide acceptance for its antifungal activities against various Candida strains.
The present study suggests that flavonoid components of pomegranate peel have the potential for prevention and treatment of Candida infections. Among the various tested extracts, sour malas peel extracts showed maximum antifungal activity. Further investigations on the antifungal potential of pomegranate preparations and in vivo investigations are needed to identify and isolate the active compounds present in pomegranate’s peel to replace the synthetic drug and additives with these natural plant-based products and also to confirm these effects in vivo.