1. Background
In general, rapid absorption from mucous routes is observed because of thin mucus membrane and rich blood supply. After absorption, drug is transferred by the deep lingual vein or facial vein and then drains into the general circulation via the jugular vein, bypassing the liver and thereby sparing the drug from first-pass metabolism. The term ‘mucoadhesive’ describes materials that bind to biological substrate, such as mucosal membranes. Adhesion of mucoadhesive drug delivery devices to mucosal membranes increases drug concentration gradient at the absorption site and therefore improves the bioavailability of systemically delivered drug (1). Polysaccharides polymers such as alginates have been investigated in the last years as carrier for controlled drug release (2, 3), cell encapsulation (4), tissue engineering material (5), or taste masking in pediatric formulations (6). Encapsulation property of alginate is due to its ability to move from sol to gel state by ionotropic gelation under mild conditions through interactions with bivalent or trivalent cations (7). The resulting particles are able to protect drugs from environmental stress or, based on alginate pH dependent solubility, from chemical/enzymatic digestion in gastric fluids. Moreover, mucoadhesive properties of alginate may increase residence time and reduction of drug metabolism (8, 9). However, physical properties (mechanical strength, wettability, pore size and distribution) of particles are strongly affected by both polymer characteristics and production technology as well as curing/drying step (10-12). In general, microparticles have the potential to be used for targeted and controlled-release drug delivery, but coupling of mucoadhesive properties to microspheres has additional advantages including efficient absorption, a much more intimate contact with the mucus layer and specific targeting of drug to the absorption site. Alginate based beads are commonly manufactured by dropping drug-polymer solutions into a bivalent cations aqueous solution. This mild and easy scalable microencapsulation technique is based on the breaking apart of a laminar jet of polymer solution into a row of mono-sized drops using a vibrating nozzle device (10, 13) producing hydrated particles. Hydrated beads can be used as self-consistent dosage form or as building blocks in the production of controlled drug release platforms. However, beads need to be dried to stabilize the dosage forms and to avoid microbiological degradation. Therefore, the drying process has a crucial role to assure the quality of the final product (13). Low methoxy polysaccharide, pectin, with the degree of esterification less than 50% can form rigid gels by the action of calcium ions or multivalent cations, which cross-link the galacturonic acid chains of pectin to yield hydrogels stable at low pH (14). Piroxicam, an oxime derivative, is a nonsteroidal anti-inflammatory drug (NSAID) generally used to alleviate pain, inflammation, and stiffness due to arthritis. The drug is highly potent and has a long half-life of over 50 hours, which makes it suitable for once daily dosage. A single daily administration of nonsteroidal antiinflammatory drugs is widely accepted as the best regimen in chronic diseases such as degenerative osteoarthropathy and inflammatory affections of the skeletal muscles (15). Aquino et al. formulated piroxicam loaded alginate beads by using prilling/microwave tandem technique (13). A tandem technique, based on the combination of prilling and microwave irradiation assisted treatments, to produce biodegradable alginate carriers of piroxicam with different behaviors of drug controlled release. Chakraborty et al. prepared algino-pectinate mucoadhesive microspheres of Na-Alg and pectin with aceclofenac by the ionotropic gelation method using calcium chloride as a cross-linking agent. Beads of aceclofenac were produced with different concentrations of polymers (14). Atmaram et al. produced calcium pectinate beads indomethacin. The purpose of this study was to improve the entrapment efficiency of water-insoluble drug using internal cross-linking agents (16). Arica et al. aimed to develop ibuprofen-loaded alginate beads as a controlled release oral delivery system in vitro and to investigate gastrointestinal side effects in vivo. In vivo data showed that administration of ibuprofen in alginate beads prevented gastric lesions (17).
2. Objectives
In this study, Piroxicam-loaded gastroretentive emulsion gel beads of algino-pectinate, were developed using hydrophilic polymers. The release behavior of gel beads capable of protective in gastric fluid was investigated to achieve a gastroretentive, multiple units and controlled release formulation of piroxicam.
3. Materials and Methods
Piroxicam, alginate sodium (Alg-Na), pectin, acetone, isopropyl alcohol, chloride calcium, potassium dihydrogenphosphat, sodium hydroxide, potassium chloride and formalin were obtained from Merck (Darmstadt, Germany). All solvents and reagents were of analytical grade.
3.1. Experimental Methods
3.1.1. Method of Preparation of Microspheres
3.1.1.1. Preparation of Na-Alginate Microspheres
Na-alginate microspheres were prepared by ionic cross-linking. Sodium alginate was dissolved in distilled water to obtain different concentrations (100, 200 and 300 mg). Piroxicam (40 mg) was added to the sodium alginate solution and dispersed with ultrahomogenizer (Heidolph, Germany) for 2 minutes at 8000 rpm. This dispersion was added dropwise to a calcium chloride solution (5 %, w/w) using a 24 G needle at a constant rate and under continuous stirring at 200 rpm. Stirring was continued for 30 minutes, for complete reaction. After 30 minutes, microspheres were collected by filtration, washed extensively with distilled water, and dried overnight at room temperature (Table 1).
| Emulsion, O/W | |||||||
|---|---|---|---|---|---|---|---|
| Formulation code | Drug: Polymer ratio | Organic Phase, O | Aqueous phase, W | Ionotropic Gelation Agent | |||
| Piroxicam, mg | Acetone, mL | Na-Alg, mg | Pectin | Water, mL | CaCl2, %w/v | ||
| F1 | 1:2.5 | 40 | 2 | 100 | - | 20 | 10 |
| F2 | 1:5 | 40 | 2 | 200 | - | 20 | 10 |
| F3 | 1:7.5 | 40 | 2 | 300 | - | 20 | 10 |
| F4 | 1:2.5:5 | 40 | 2 | 100 | 200 | 20 | 10 |
| F5 | 1:5:5 | 40 | 2 | 200 | 200 | 20 | 10 |
| F6 | 1:7.5:5 | 40 | 2 | 300 | 200 | 20 | 10 |
| F7 | 1:7.5:2.5 | 40 | 2 | 300 | 100 | 20 | 10 |
| F8 | 1:7.5:7.5 | 40 | 2 | 300 | 300 | 20 | 10 |
2.1.1.2. Preparation of Algino-Pectinate Microspheres
Pectin and Na-alginate solutions of different concentrations were separately prepared by dissolving both polymers in water under gentle agitation. Pectin solution was then added into Na-alginate solution and the mixture was stirred at 4000 rpm. Then, Piroxicam (40 mg) was added to the polymer solution and dispersed with ultrahomogenizer for 2 minutes at 8000 rpm. The remaining procedure was the same as Na-alginate microspheres (Table 1).
3.2. Determination of Encapsulation Efficiency of Alginate and Algino-Pectinate Beads
Twenty milligrams of the beads were accurately weighed, diluted with phosphate buffer (pH = 6.8) to 10 mL each and placed in stirrer for three hours. Aliquot samples of 10 mL were removed through a filter and assayed spectrophotometrically (Shimadzu UV-160 A, Japan) at 347.6 nm. The determination was performed in triplicate and the mean drug encapsulation efficiency was calculated.
3.3. Frequency Distribution Analysis
Microparticle samples were analyzed for frequency distribution with calibrated optical microscope, fitted with a stage and an ocular micrometer. Small quantities of microsphere were spread on a clean glass slide and the average size 60 particles, frequency distribution were determined in each batch using scion image and sigma plot (Table 1).
3.4. Percentage Yield Value
The percentage yield value was defined as the quantity of beads produced as a function of loaded drug and polymer.
3.5. Differential Scanning Calorimetry (DSC)
Differential scanning calorimeter (Shimadzu, Japan) was used to monitor thermal events during heating. Samples weighing 5 mg were placed in open aluminium pans and heated from 25 to 300 °C at a rate of 10°C/min.
3.6. Flow Property Study
Bulk density and tapped densities were determined using density apparatus. The Carr’s index (%) and the Hausner’s ratio were calculated using the bulk density and tapped density. The angle of repose of untreated piroxicam powder and the microparticles were assessed by fixed funnel method.
3.7. Surface pH Measurement
Surface pH of microparticles was determined to evaluate possible irritation to gastric mucosa. Microparticles were allowed and swell with 50 mL of 0.1 M HCl buffer (pH = 1.2, 2 hours) and phosphate buffer (pH 6.8, 6h) and pH was measured at time intervals of 0, 1, 2, 4, 6 and 8 hours using glass electrode in contact with microparticles and microparticles on pH meter (Corning pH meter 120, USA).
3.8. Swelling Study
Swelling of the beads was studied in triplicate using three randomly selected beads from each batch. Beads of known mass were placed in wire basket containing 50 mL of 0.1 M HCl buffer (pH = 1.2, 2 hours) and phosphate buffer (pH = 6.8, 6 hours) maintained at 37°C. The beads were periodically removed at predetermined time intervals during the study period of 8 hours, drained on tissue paper and weighed.
3.9. Measurement of Mucoadhesive Strength Exvivo
Mucoadhesive strength of piroxicam discs was measured using modified physical balance by the method described by Wong et al. (12). Tissue cut from mucosal abdominal of rat (hairless or cut the hair) was used as the model substrate and phosphate buffer pH = 6.8 was used as the moistening fluid. Male Wistar rats (260 ± 30 g) were used in this study. The animals were given food and water ad libitum. They were housed in the Animal House of Tabriz University of Medical Sciences at a controlled ambient temperature of 25 ± 2°C with 50 ± 10% relative humidity and a 12-h light/12-h dark cycle. The present study was performed in accordance with the Guide for the Care and Use of Laboratory Animals of Tabriz University of Medical Sciences, Tabriz-Iran (The National Institutes of Health Publication No 85-23, revised 1985). Mucosa pieces were stored frozen in phosphate buffer pH = 7.4 and thawed to room temperature before use (18). At the time of testing, a section of mucosa was secured to the upper glass vial using a cyanoacrylate adhesive. The diameter of each exposed mucosal membrane was 2 cm.
3.10. Exvivo Residence Time
The selected batch was subjected to exvivo mucoadhesion test. The disintegration medium contained 900 mL HCl buffer pH = 1.2 maintained at 37°C and after 2 hours, added 17 mL phosphate buffer stock (pH 6.8) for 6 hours. A segment of abdominal mucosa of rat, 3 cm long, was glued to the surface of a glass slab, vertically attached to the apparatus. The mucoadhesive discs were hydrated from one surface and then brought into contact with the mucosal membrane. The glass slab was vertically fixed to the apparatus and allowed to move up and down, so that the disc was completely immersed in the buffer solution at the lowest point and was out at the highest point. The time necessary for complete erosion or detachment of the discs from the mucosal surface was recorded. The experiment was performed in triplicate.
3.11. Histology
After removal of abdominal mucosa of rat from residence time test, the tissues were placed in 10% buffered formaldehyde solution and fixed for 72 hours. Afterwards, tissues were processed according to routine light microscope technique. First, they were dehydrated in ascending degrees of ethyl alcohol (70, 80, 90, 96, 99%) and then cleared in xylene and embedded in paraffin. Five-micrometer paraffin sections were cut and stained with hematoxylin-eosin, examined and photographed with microscope.
3.12. In vitro Release Studies
Release of piroxicam from alginate beads was performed using a bath-shaker. The weighed amounts of piroxicam-loaded samples were put into a glass vessel. The dissolution mediums (900 mL) were 0.1 N HCl solution (pH = 1.2) and phosphate buffer solution (pH = 6.8). The glass vessel was then immersed into the water bath. Temperature of the dissolution vessel was maintained at 37 ± 0.5°C. An aliquot of 5 mL sample was withdrawn at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8 and 24 hours intervals and similar volume was replaced with fresh phosphate buffer (pH = 6.8) maintained at same temperature and the amount of piroxicam was determined by spectrophotometer (Shimadzu UV-160 A, Japan) at 347.6 nm. All studies were performed in triplicate. To have a better comparison between different formulations dissolution efficiency (DE), t50% (dissolution time for 50% fraction of drug) and difference factor, f1 (used to compare multipoint dissolution profiles) were calculated. Dissolution efficiency (DE as the area under the dissolution curve up to a certain time, t, expressed as a percentage of the area of the rectangle arising from 100% dissolution in the same time) was calculated using Equation 1 (19):
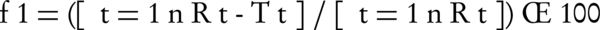
Where n is the number of time points at which percentage dissolved was determined, Rt is the percentage dissolved of one formulation at a given time point and Tt is the percentage dissolved of the formulation compared at the same time point. The difference factor fits the result between 0 and 15 when the test and reference profiles are identical, and approaches above 15 as the dissimilarity increases.
3.13. Statistical Analysis
Results were evaluated using One-way ANOVA at 0.05 levels and Using SPSS version 18 (IBM, USA).
4. Results
4.1. Physicochemical Properties of Microparticles/Discs
Mucoadhesive enteric microparticles of piroxicam were prepared using Na-Alg. Microparticles physical characteristics were characterized (Table 2). The production yield of the beads increased (82.57-90.8%) with increase in the concentration of polymer (Na-Alg). The production yield of beads prepared in the algino-pectinate varied from 79.68-91.81% (Table 2). The encapsulation efficiency of surface cross-linked beads increased with an increase in the pectin concentration (F6 to F8), but decreased with increasing the Na-Alg concentration (Pectin with constant concentration) from F4 to F6. According to Table 2, by increasing the concentration of Na-Alg (F1 to F3), the mean particle size of beads increased (945.4-1058.3 µm). Larger beads were obtained by increasing the concentration of sodium alginate (F3, 1:7.5 ratio). On adding pectin, the particle size was increased when increasing the concentration of F4 to F8 (Table 2). The encapsulation efficiency was low for F1 to F3 formulations (with increasing the Na-Alg concentration), but increased with an increase in the pectin concentration (F4 to F8). The highest loading efficiency and particle size found for F8 formulation (with 300 mg Na-Alg and 300 mg Pectin concentration) were 66.72% and 11024 µm, respectively. Data analysis showed that all obtained microparticles followed a log-probability distribution.
| Formulation Code | Drug: Polymer Ratio | Production Yield, % | Theoretical Drug Content ,% | Drug Entrapped, % | Drug Loading Efficiency, % | Particle Size, µm |
|---|---|---|---|---|---|---|
| F1 | 1:2.5 | 82.57 | 28.57 | 8.23 ± 0.04 | 28.8 ± 0.12 | 945.4 ± 12.02 |
| F2 | 1:5 | 87.91 | 16.66 | 5.74 ± 0.01 | 34.45 ± 0.08 | 940.11 ± 11.61 |
| F3 | 1:7.5 | 90.8 | 11.76 | 2.36 ± 0.006 | 20.06 ± 0.02 | 1058.3 ± 11.80 |
| F4 | 1:2.5:5 | 82.35 | 11.76 | 5.89 ± 0.26 | 50.01 ± 2.26 | 899.91 ± 12.02 |
| F5 | 1:5:5 | 89.77 | 9.09 | 3.63 ± 0.005 | 39.93 ± 0.06 | 1117.8 ± 11.48 |
| F6 | 1:7.5:5 | 88.88 | 7.4 | 3.86 ± 0.006 | 52.16 ± 0.07 | 1012.81 ± 11.27 |
| F7 | 1:7.5:2.5 | 91.81 | 9.09 | 4.02 ± 0.030 | 44.21 ± 0.3 | 1054.4 ± 10.15 |
| F8 | 1:7.5:7.5 | 79.68 | 6.25 | 4.17 ± 0.010 | 66.72 ± 0.18 | 1102.4 ± 10.25 |
a Data are presented as Mean ± SD.
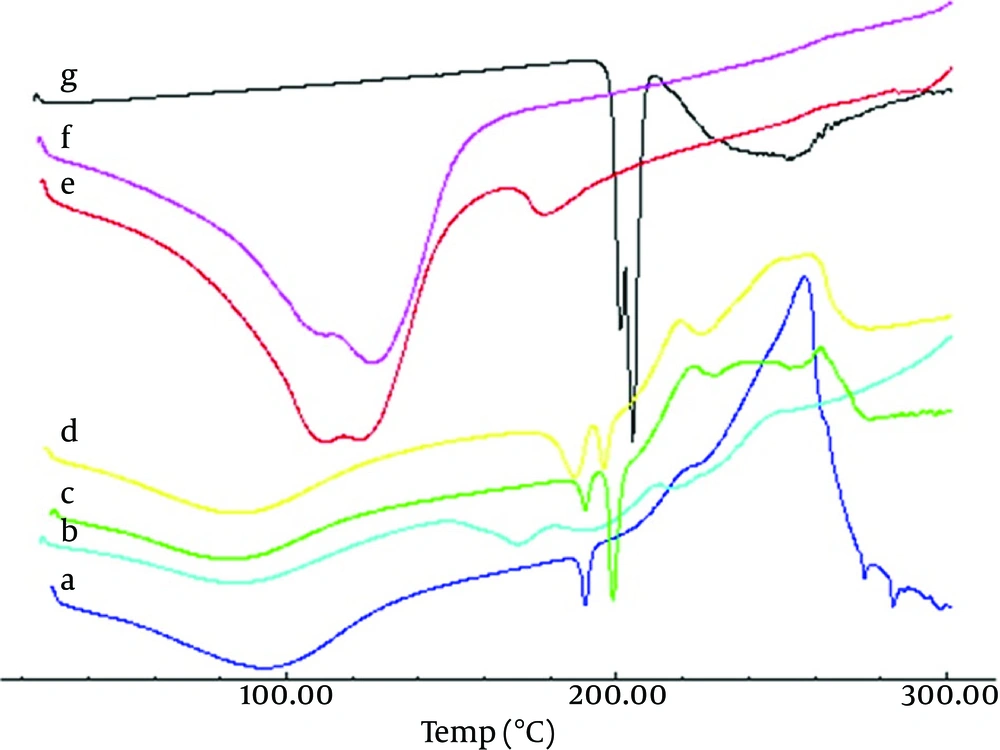
Angle of repose for microspheres was between 16.18° and 18.25°, thus indicating good flow property for microspheres (Table 3). DSC curves of the piroxicam (Figure 1) showed an endothermic peak around 193.88-210.05°C, which corresponds to its melting point. However, in the thermogram of the microparticles the endothermic peak corresponds to Na-Alg melting, but drug and pectin peaks were absent, suggesting the amorphous state of the drug in the microparticles. This conversion happened during the preparation of microparticles. Physical mixture of microparticles showed endothermic peak of piroxicam, and pectin peak might be overlapped with peak of Na-Alg in the thermograms.
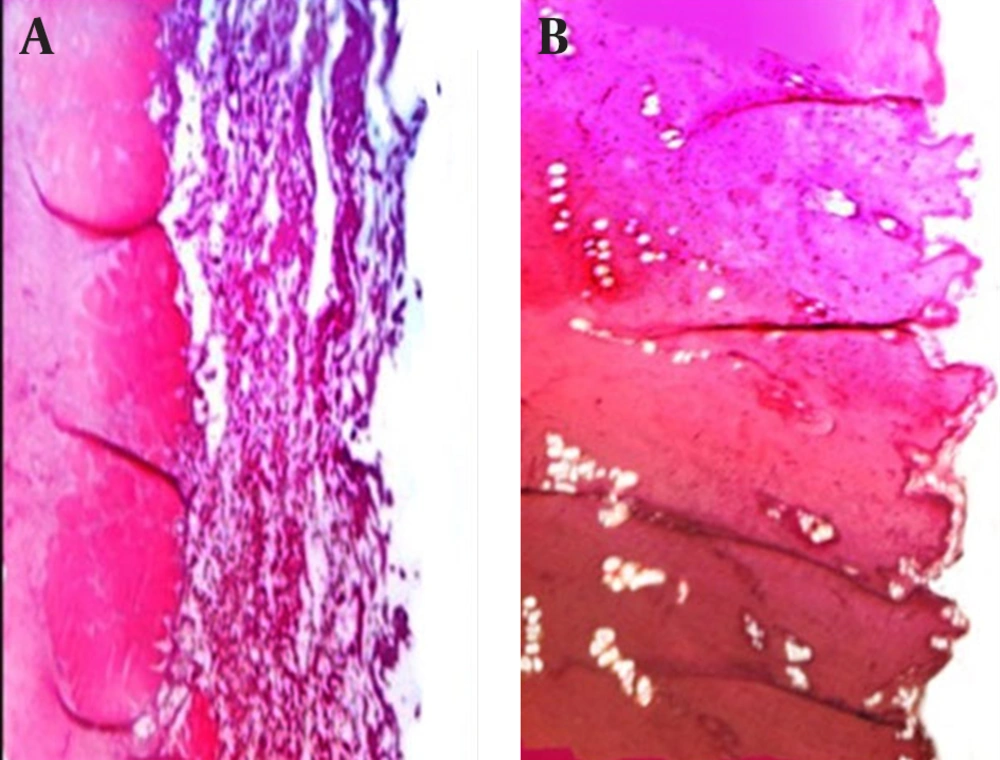
The discs of all formulations had good appearance: 100.63-103.8% weight variation, 12.8-28 N hardness, and 0.84-9.05% friability (Table 3). The surface pH of all microparticles were in the range of 1.59-1.61 (pH = 1.2 in stomach) to 7.04-8.40 (pH = 6.8 in intestine). For discs F1 to F3 (prepared with Na-Alg polymer) and F4 to F8 (mixture of Na-Alg-Pectin polymers) ranged 1.060 to 1.271, which was in accordance with the referred value of 1.2 in stomach, but for discs F1 to F3 (7.37-7.89) and F4 to F8 (7.04-8.4) did not agree the referred value 6.8-7.4 in intestine (Table 5). Table 3 showed the percentage swelling of different microparticles formulations at different time intervals. It was revealed that all microsphere formulations swelled rapidly when immersed in 0.1 M phosphate buffer (pH = 6.8). In vitro residence time with rat abdominal mucosa in simulated gastric (pH = 1.2, 2 hours) and (pH = 6.8, 6 hours) varied for microparticles from 0 to 480 minutes (Table 3). Microparticles (F4 to F8) showed highest mucoadhesion in this study (180-480 minutes), and did not dissolve in 0.1 M HCl about 2 hours. For F1 formulation, time of microparticles remaining was 30-120 minutes. In contrast, microparticles containing mixture Na-Alg-Pectin polymers showed relatively higher retentive than formulations of prepared with single Na-Alg polymer. In vitro mucoadhesive strength study was performed and the results were shown in Table 3. On the modified physical balance and measure the force (g/cm2) required detaching the disc. The mucoadhesion characteristics were affected by the concentration of mucoadhesive polymers. The formulations (F1, F2 and F3) with Na-Alg showed the mucoadhesive strengths of 3.29, 4.81 and 6.56 g/cm2, respectively. Higher concentration of polymer (Na-Alg) increased mucoadhesive strength of formulation. F6 Formulation containing 1:12.5 ratio (drug: Na-Alg and Pectin) showed the highest mucoadhesion (11.14 ± 0.72 g/cm2). Microscopic observations revealed no significant effect of nanoparticles on the microscopic structure of mucosa. As shown in Figure 2, no cell necrosis was observed.
| Formulations | Drug: Polymer/s, Alg/Alg-Pectin Ratio | Bulk Density, g/cm3 | Tapped Density, g/cm3 | Carr’s index, % | Hausner Ratio | Angle of repose, °θ |
|---|---|---|---|---|---|---|
| F1 | 1:2.5 | 0.23 ± 0.02 | 0.26 ± 0.02 | 11.54 ± 0.00 | 1.13 ± 0.01 | 19.29 ± 0.32 |
| F2 | 1:5 | 0.64± 0.02 | 0.91 ± 0.01 | 29.70 ± 0.01 | 1.42 ± 0.02 | 9.09 ± 0.14 |
| F3 | 1:7.5 | 0.76 ± 0.01 | 0.82 ± 0.02 | 7.32 ± 0.00 | 1.08 ± 0.02 | 5.71 ± 0.76 |
| F4 | 1:2.5:5 | 0.55 ± 0.02 | 0.59 ± 0.01 | 6.8 ± 0.00 | 1.07 ± 0.00 | 21.8 ± 0.25 |
| F5 | 1:5:5 | 0.77 ± 0.03 | 0.88 ± 0.03 | 12.5 ± 0.02 | 1.14 ± 0.01 | 9.09 ± 0.1 |
| F6 | 1:7.5:5 | 0.81 ± 0.05 | 0.86 ± 0.02 | 5.8 ± 0.00 | 1.06 ± 0.02 | 4.9 ± 0.05 |
| F7 | 1:7.5:2.5 | 0.89 ± 0.06 | 1.01 ± 0.04 | 11.9 ± 0.03 | 1.13 ± 0.01 | 11.3 ± 0.21 |
| F8 | 1:7.5:7.5 | 0.71 ± 0.04 | 0.75 ± 0.03 | 5.33 ± 0.00 | 1.05 ± 0.00 | 8.27 ± 0.2 |
| Piroxicam (untreated) | - | 0.23 ± 0.01 | 0.41 ± 0.01 | 43.90 ± 0.03 | 1.80 ± 0.00 | 36.87 ± 0.68 |
a Data are presented as Mean ± SD.
| Formulation code | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 |
|---|---|---|---|---|---|---|---|---|
| Polymer/s, Alg/Alg-pectin ratio | 1:2.5 | 1:5 | 1:7.5 | 1:2.5:0.5 | 1:5:5 | 1:7.5:5 | 1:7.5:2.5 | 1:7.5:7.5 |
| Weight variation, mg | 100.63 ± 0.5 | 107 ± 5.3 | 103.16 ± 1.8 | 103.8 ± 5.5 | 101.8 ± 0.4 | 100.8 ± 0.7 | 103 ± 2.1 | 102.9 ± 1.8 |
| Hardness, N | 28 ± 9.2 | 19.6 ± 5.32 | 22.1 ± 1.9 | 21 ± 3.5 | 24.5 ± 1 | 12.8 ± 2 | 19.6 ± 2 | 21 ± 1 |
| Friability, % | 1.55 ± 0.33 | 1.22 ± 0.23 | 0.84 ± 0.15 | 9.05 ± 0.70 | 1.71 ± 0.08 | 2.15 ± 0.11 | 6.98 ± 1.36 | 1.96 ± 0.51 |
| Content uniformity, % | 70.70 ± 4.62 | 61.80 ± 4.20 | 68.03 ± 5.20 | 79.93 ± 6.62 | 88 ± 7.20 | 78.37 ± 4.45 | 82.5 ± 4.78 | 89.60 ± 5.23 |
| pH surface | 7.89 ± 0.28 | 7.37 ± 0.57 | 7.50 ± 0.30 | 7.32 ± 0.51 | 7.04 ± 0.19 | 8.40 ± 0.55 | 8.31 ± 0.45 | 8.23 ± 0.39 |
| Swelling, %a | 73.16 ± 5.15 | 61.17 ± 17.42 | 74.95 ± 14.17 | 54.15 ± 7.18 | 58.02 ± 2.24 | 64.83 ± 20.05 | 53.17 ± 12.1 | 59.67 ± 9.86 |
| Mucoadhesive strength, g/cm2 | 3.29 ± 0.59 | 4.81 ± 0.69 | 6.56 ± 0.54 | 7.82 ± 0.59 | 11.14 ± 0.72 | 5.87 ± 0.45 | 4.53 ± 0.63 | 5.20 ± 1.29 |
| Residence time, min | 120 ± 0.3306 | 60 ± 0.00 | 30 ± 0.00 | 480 ± 60 | 180 ± 30 | 180 ± 30 | 240 ± 30 | 480 ± 120 |
aAll of the results are related to the eight hour.
4.2. Release study
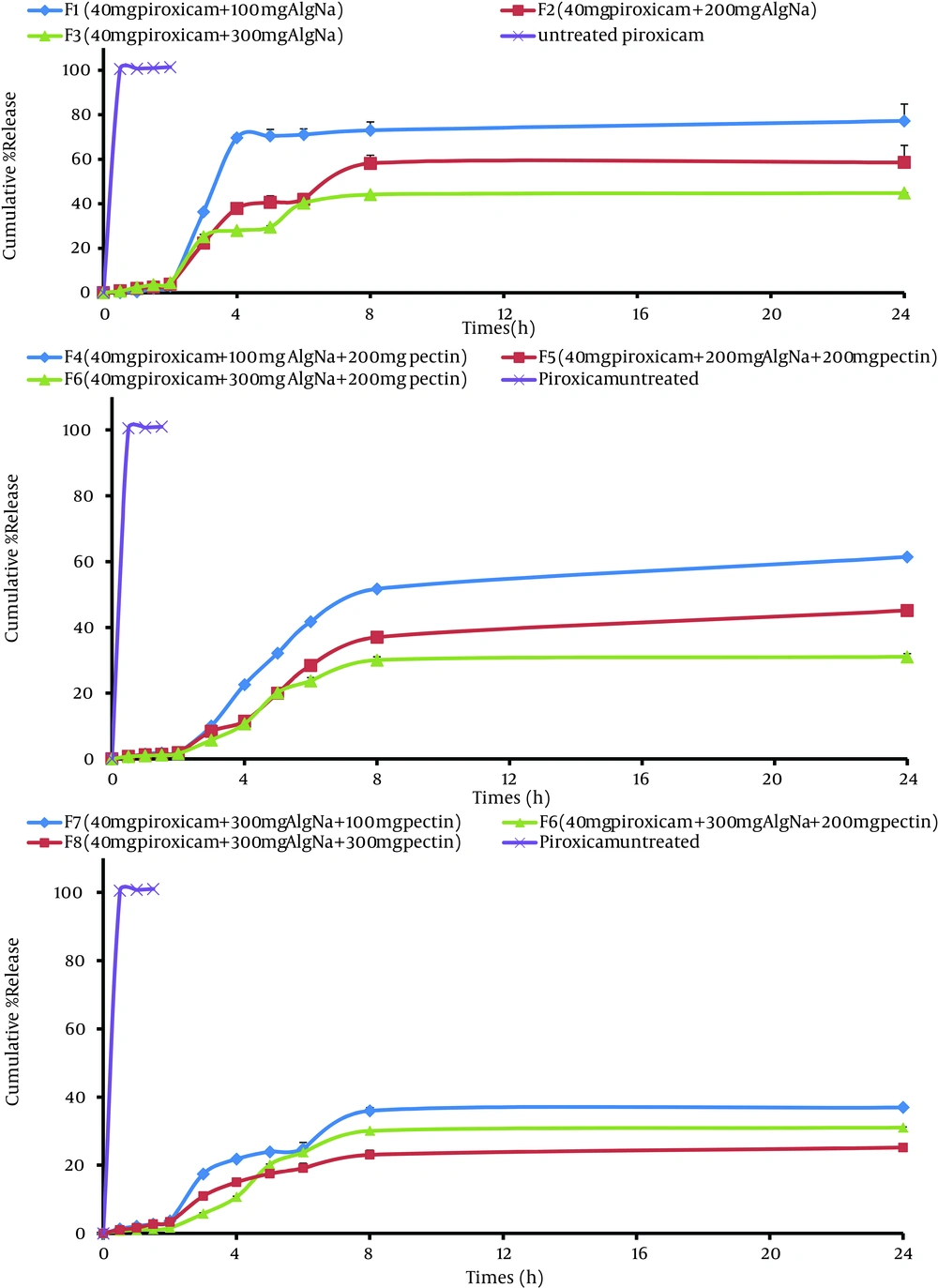
The effect of polymer level on the release of piroxicam from the microparticles was studied (F1-F8). Formulations F1, F2 and F3 were able to sustain the drug release for 8 hours; whereas, algino-pectinate microparticles (F4 to F7) were able to sustain the drug release for more than 24 hours (Figure 3Table 5). By increasing the quantity of pectin up to 300 mg, drug release was too slow and only 23.02-51.70% of the drug was released after 8 hours (P > 0.05). Rel8 for F1, F4 and F6 were 73.07%, 51.70% and 30.06%, respectively. Formulations containing Na-Alg alone (F1 to F3) underwent erosion before occurrence of complete swelling, resulting in faster drug release.
Piroxicam exists in its acidic form, which is practically insoluble in the stomach pH (14). Due to this fact and in an attempt to reduce adverse effects, the enteric nature of microspheres was evaluated to find out whether the prepared formulations were able to restrict the drug release in an acidic environment. Na-alginate microspheres and algino-pectinate microspheres were able to restrict drug release in acidic medium, but some amounts of drug was found to be released at stomach pH. It was observed that a much lower amount of piroxicam was released in the acidic environment (3.04-28.94 % drug) from Na-alginate microspheres in 0.1 M HCl buffer medium, pH = 1.2, in 2 hours, but 1.59-3.36% in the case of algino-pectinate microspheres (P > 0.05). This may be attributed to the fact that piroxicam present on the surface of microspheres, immediately encounters the medium. Therefore, these formulations could protect the acid-labile drug and enzyme from gastric degradation (14). The coefficient of determination (R2) was used as an indicator of the best fitting for each of the considered models (Table 6). Kinetic data of all formulations reached a higher coefficient of determination (R2 = 0.863-0.955) with the Korsmeyer-Peppas model; whereas, the release exponent value (n) ranged from 0.527 to 0.918 (Table 6).
| Formulation | Rel2, % | Rel8, % | DE | T50%, min | f1 |
|---|---|---|---|---|---|
| F1 | 3.04 ± 0.36 | 73.07 ± 0.99 | 65.09 | 226.78 | 55.72 |
| F2 | 15.57 ± 11.44 | 58.18 ± 3.69 | 48.42 | 254.01 | 70.16 |
| F3 | 28.94 ± 9.57 | 44.15 ± 0.13 | 37.74 | 230.58 | 75.23 |
| F4 | 1.96 ± 0.04 | 51.70 ± 0.25 | 45.29 | 381.31 | 75 |
| F5 | 1.92 ± 0.26 | 37.01 ± 0.15 | 32.47 | 298.90 | 82.74 |
| F6 | 1.59 ± 0.26 | 30.06 ± 1.08 | 24.72 | 298.90 | 86.06 |
| F7 | 3.36 ± 0.34 | 23.02 ± 1.34 | 30.18 | 270.82 | 81.10 |
| F8 | 3.26 ± 0.34 | 23.02 ± 1.34 | 20.22 | 293 | 86.77 |
| Piroxicam untreated | 101.42 ± 5.37 | 102.12 ± 4.98 | 96.86 | 45.22 | 0 |
aAbbreviations: Rel2, amount of drug release after 2 hours; Rel8, amount of drug release after 8 hours; DE, dissolution efficiency; t50%, dissolution time for 50% fractions; f1, differential factor (0 < f1 < 15).
b Data are presented as Mean ± SD.
| Formulation | Order | MPE, % | RSQ | Slope | Intercept | K |
|---|---|---|---|---|---|---|
| F1 | Peppas | 40.59 | 0.955 | 3.315 | -18.826 | 0.000 |
| F2 | log-probability | 50.93 | 0.871 | 0.849 | -5.382 | 0.0001 |
| F3 | linear-probability | 40.53 | 0.863 | 0.720 | -4.802 | 0.0002 |
| F4 | Peppas | 35.92 | 0.938 | 1.801 | -11.683 | 0.000 |
| F5 | reciprocal powered time | 40.624 | 0.907 | -1.495 | 10.339 | 0.0001 |
| F6 | reciprocal powered time | 49.104 | 0.863 | -1.406 | 10.112 | 0.0001 |
| F7 | log-probability | 39.122 | 0.869 | 0.615 | -4.405 | 0.0003 |
| F8 | log-probability | 32.775 | 0.888 | 0.527 | -4.140 | 0.0004 |
aAbbreviations: MPE, mean percent error; K, Kinetic Constant.
5. Discussion
Hydrogels can be defined as three-dimensionally cross-linked polymer chains able to hold water within their porous structure. Cross-linking or apparent gelation of calcium matrices of the hydrophilic polymers occur rapidly enclosing the drug, increasing the entrapment efficiency, but further rearrangement of gel structure continued for a long period (20). Increased entrapment efficiency with an increase in pectin concentration at constant drug amount may be attributed to the availability of excess polymer to encapsulate the drug. Reduction in entrapment efficiency may be attributed to the weakening of surface gel strength due to an excess of Ca+2 ions for the formation of “egg-box”. This is caused by pregelation, a rapid reaction between pectin molecules and calcium ions, resulting in nonhomogeneous gel structure (20). Increasing particle size may be due to higher polymer concentration (Na-Alg/Na-Alg-Pectin), which increases the viscosity of medium and makes greater availability of Ca+2 binding sites in polymeric chains. As a result, the degree of cross-linking increased and larger droplets were formed, entrapping a higher amount of drug. The best drug to polymer ratio in microparticles was 1:2.5 (F1) with Na Alg and 1:7.5 (F4) with Alg Na with pectin, respectively. Microparticles F1 and F4 showed 28.80%, 50.01% loading efficiency, 82.57%, 82.31% production yield and 945.4, 899.91 µm mean particle size.
The friability of all formulations (discs) was higher than 1%, ensuring that the discs were not mechanically stable (Table 3). The findings were supported by Carr’s (compressibility) index, which was < 20, indicating good flow characterizes (except F2). The Hausner ratio is correlated to flowability of a powder or microparticles. A Hausner ratio smaller than 1.25 is considered as an indication of good flowability (except F2, 1.42). Micrometric study showed that the prepared microparticles had a good flow property, excellent compressibility, and good packability compared to the pure drug. DSC analysis of microparticles revealed that any abrupt or drastic change in the thermal behavior of either the piroxicam with polymers (Na-Alg and Pectin) may indicate a possible drug-polymer interaction (Figure 1). Microparticles prepared with mixture Na-Alg and pectin polymers change pH to more than the basic value (Table 3). All microspheres exhibited good mucoadhesive properties. Both Na-alginate and algino-pectinate microspheres had less mucoadhesion in simulated gastric fluid (pH = 1.2) compared to simulated intestinal fluid (pH = 6.8). This is probably because Na-alginate is practically insoluble in aqueous acidic solution; whereas, its solubility, hydration and bioadhesion properties were increased in simulated intestinal fluid due to ionization of the carboxyl acid group and other functional groups present in the polymer. This increased solubility allowed more solvent to penetrate the polymeric coat, producing a viscous gel and increasing the mucoadhesion property. It was also observed that algino-pectinate microspheres had a better mucoadhesive property (Table 3) than alginate microspheres, because a combination of both polymers increases the viscosity of the matrix, which helps in increasing adhesion to the intestinal mucosa. Adhesion of microspheres to the intestinal mucosa for a prolonged period is suggested where they release the drug in a sustained manner. Furthermore, drug release was extremely slow at acidic pH and increased in phosphate buffer. This is because alginate microspheres shrink at acidic pH due to the tightening of gel meshwork, whereas at pH = 6.8 alginate erodes and releases the contents in a sustained manner (14). Algino-pectinate microspheres (F5 to F8) were more efficient in sustaining the drug release compared to alginate microspheres (F1-F3), because pectin could form a rigid coat with calcium ions, thus forming a calcium-pectinate gel matrix. Free carboxyl groups in pectin molecule are distributed block-wise; therefore, pectin gelation is possible because of the “egg-box” structure, resulting from chelation of calcium ions in electronegative cavities formed by the carboxyl residues and hydroxyl groups (14). The release exponent in the Korsmeyer-Peppas model suggested that the mechanism leading to the release of piroxicam was an anomalous non-Fickian diffusion mechanism, indicating that a combined release mechanism of drug diffusion and sphere erosion might be appropriate. Similar to our investigation, Nochodchi et al. (20) reported that as there is more Ca+2 to bind, a better and stronger gel is formed around the matrix and this strong gel does not allow the dissolution medium to penetrate the matrix at a high speed, resulting in a reduction in release rate (t50% = 226.78-381.21 minutes). In conclusion, alginate and algino-pectinate formulations exhibited promising properties of a controlled release form for piroxicam. Theoretical models indicated that drug release from the microparticles was triggered by matrix disintegration followed by swelling and relaxation of the polymer chain. In vivo data showed a significant difference for percent inhibition when the optimized formulation was compared with the pure drug.