1. Background
Aflatoxins are secondary toxic metabolites and found in most plant products including peanuts, copra, soya, maize, rice and wheat. Aflatoxins B1, B2, G1 and G2 are primarily produced by special strains of Aspergillus flavus and Aspergillus parasiticus (1). Aflatoxin B1 (AFB1) is the most common and toxic one. The levels of aflatoxins in food could be increased under conditions of high humidity (2). Therefore, the contamination levels of AFM1 were found more commonly in spring than winter (3). AFM1 is a metabolite of AFB1 that could be found in milk when dairy cattle are fed with contaminated feedstuff (4). It is relatively stable to heat and is not degraded during pasteurization process (5). Aflatoxins have shown to be immunosuppressant, mutagen, teratogen and carcinogen to animals and humans (6, 7). International Agency for Research on Cancer (IARC) and the World Health Organization (WHO) have classified AFB1 and M1 as primary and secondary groups of carcinogenic compounds, respectively (8). Milk is a major nutrient for infants, children and adults. Infants are usually fed with milk as the main source of food, so the problem is more serious in this group of users (9). Since milk is used for the production of infant formula, yogurt and cheese, determination of AFM1 concentration in milk and dairy products, to inform consumers of its potential risk, is of utmost importance. As one of the quality and safety aspects of dairy products, AFM1 in milk is monitored and regulated in most countries (1). The maximum tolerance level of AFM1 concentration in milk is 50 ng/L in the European countries (10). Many studies have shown a high incidence of AFM in Iran (11-14).
2. Objectives
There have been several studies on AFM1 concentration in milk samples in different regions of the world and also in Iran, but this study aimed to determine the current status of AFM1 contamination in raw milk samples of Fariman city, located Khorasan Razavi province, Iran.
3. Materials and Methods
Fariman is a small city in Northeast Iran. There are only a few small local dairies in the city with an average of 60 cows for each dairy. A total of 45 raw cow milk samples from dairy farms of Fariman city were collected randomly (15 samples every month) during the summer (July to September 2012). The quantity of AFM1 was measured using commercially available competitive ELISA® kits (Ridascreen AFM1, R-biopharm, Germany) according to the manufacturer’s protocol. Milk samples were centrifuged at 10°C for 10 minutes in 3500 rpm for degreasing. After centrifugation, upper cream layers were completely removed by aspirating through a Pasteur pipette and the lower phases were used for the quantitative analysis. AFM1 standard solutions were used for the construction of calibration curve at concentrations of 5, 10, 20, 40 and 80 ng/L. One-hundred microliters of the standard solutions or the defatted samples were added to the wells to occupy the binding sites, then mixed gently and incubated for 60 minutes at room temperature. The liquid was poured out of the wells and the wells filled with 250 µL washing buffer and poured the liquid out again. This washing step was repeated four times. Then, 100 µL of the diluted enzyme conjugate was added and incubated for 30 minutes at room temperature in the dark and repeated washing again. In the next stage, 100 µL of substrate/chromogen was added to the wells and incubated for 15 minutes at room temperature in the dark. Attached enzyme conjugate converted the chromogen to a blue product and then 100 µL of the stop solution was added to the wells leading to a color change from blue to yellow. Lastly, the absorbance was measured at 450 nm by a microplate ELISA reader within 30 minutes.
3.1. Statistical Analysis
Data was statistically analyzed using Student’s t-test to determine significant differences among the three months. Statistical tests were performed using INSTAT software (GraphPad, Inc., San Diego, CA). P value less than 0.05 was considered statistically significant. The values are expressed as Mean ± SEM.
4. Results
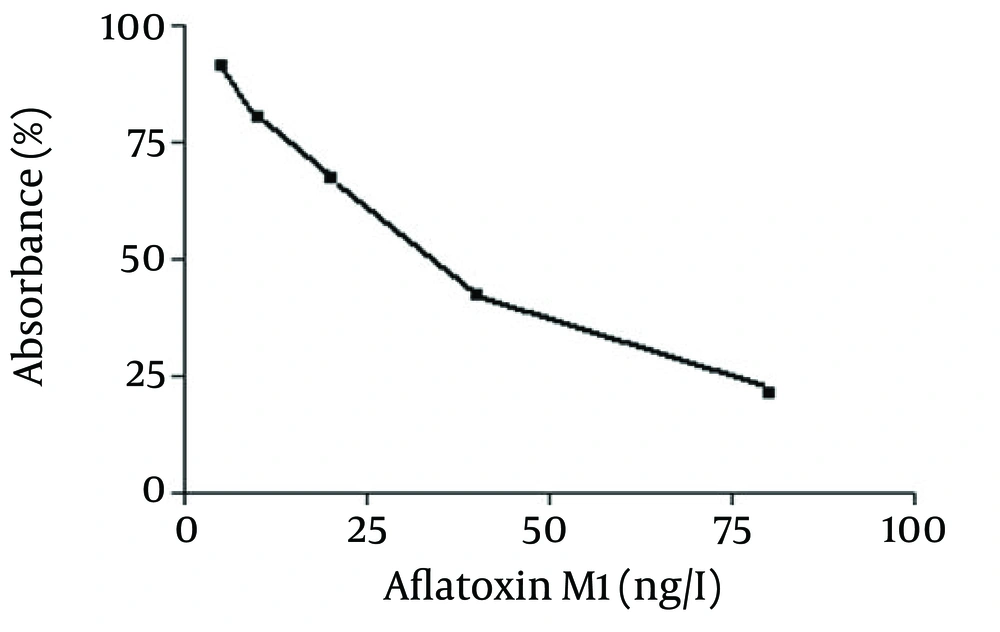
The calibration curve for AFM1 detection by ELISA analysis is shown in Figure 1. As can be seen, the standard curve was found linear over the 5 - 80 ng/L range. The detection limit of the analysis was 5 ng/L. In this cross-sectional study, AFM1 was found in all milk samples with a range of 6.3 - 23.3 ng/L. Mean and SEM as well as the ranges of AFM1 concentrations in pasteurized milk samples in July, August and September 2012 were summarized in Table 1. The mean concentration of AFM1 was significantly higher (P < 0.001) in September than July. There was no significant difference (P > 0.05) between the results of August and September. None of the samples had AFM1 higher concentrations than the maximum tolerance limit (50 ng/L) recommended by the European Union. Frequency distribution of AFM1 concentrations in raw milk samples of different months of summer 2012 in Fariman are summarized in Table 2.
| Months | < 10 ng/L | 11 - 25 ng/L | 26 - 50 ng/L | > 50 ng/L |
|---|---|---|---|---|
| July | 15 (33.33) | 0 (0) | 0 (0) | 0 (0) |
| August | 8 (17.77) | 7 (15.55) | 0 (0) | 0 (0) |
| September | 0 (0) | 15 (33.33) | 0 (0) | 0 (0) |
| Total | 23 (51.12) | 22 (48.88) | 0 (0) | 0 (0) |
Frequency Distribution of AFM1 Concentrations in Raw Milk Samples of Different Months of Summer 2012 in Fariman, Iran a
5. Discussion
Aflatoxins are both acutely and chronically toxic for animals and humans and can cause dangerous diseases including acute toxic hepatitis, liver cirrhosis and hepatocarcinoma (5, 15). AFM1 contamination in dairy products is a global problem threatening public health in all areas of the world. Despite high consumption of dairy products in Iran, a few credible data is available on their contamination levels with AFM1. In a few studies performed on AFM1 in milk, it was found in many of the samples. As can be seen in Table 3 in different cities of Iran, considerable percentage of analyzed milk samples showed AFM1 contaminations higher than the European limit (16-19). Likewise, AFM1 was found in all raw milk samples of the current study in July, August and September 2012. Nevertheless, all samples collected in summer 2012 had AFM1 below 50 ng/L, which is the maximum standard level recommended by the European limit. In Fariman, all dairies use a total mixed ration for feeding dairy cattle. The most common ingredients of rations fed to dairy cows as the farmers informed us were corn silage, clover, wheat and barley straws, and the concentrates dominantly contained maize, barely, wheat bran, soybean meal, cottonseed meal and molasses. Our results showed that contamination of milk with AFM1 in Fariman is lower than other regions reported in Iran, most probably due to low contaminated feed used by dairy animals. According to the results of Table 3, AFM1 contamination in milk samples of Iran seems to be a critical problem, especially for infants and children (9). Since the AFM1 appears in milk, followed by ingestion of AFB1-contaminated feed, feedstuff quality is an essential factor in production of contaminated milk. Therefore, the wide fluctuations in AFM1 concentrations among studies could be associated with dairy cattle feed quality. On the other hand, the feed quality is affected by many factors such as geographic and climatic conditions, feeding system types and farm management practices (20). Any changes in these factors could lead to marked fluctuations in AFM1 levels in milk (21). Many authors showed that AFM1 concentrations were affected by the seasonal effect. They reported higher level of AFM1 in cold seasons compared to hot seasons (12, 22-24). The reason is that in winters milking animals are usually fed with compound feeds and thus concentration of AFB1 increases, which in turn increases AFM1 concentration in milk. In addition, humidity affects the presence of AFB1 in feeds. A. flavus and A. parasiticus can easily grow in feedstuffs having humidity between 13% and 18%, and then they are able to produce aflatoxin in environmental humidity between 50% and 60% (25). For this reason, the level of AFM1 in feed in rainy months is more than dry months, which is in agreement with our study results in September compared with the other months. This might be due to hot summer in Fariman and raining in September that increase aflatoxin production by the end of summer. Aflatoxins are highly toxic compounds, it is therefore important to minimize the health risk from AFM1 contamination in milk, which can be consumed by infants and children as the most at-risk groups (9). Therefore, dairy farmers should be educated on potential health risk of aflatoxins. AFM1 levels should be monitored as a part of quality control procedures in dairy factories. For this reason, milk and other dairy products have to be checked continuously by accurate and precise analytical methods for the presence of AFM1 contamination and quantification. Furthermore, the level of AFB1 in feed can be reduced by monitoring the cultural phases and storage practices for prevention of mould growth and aflatoxin production (26, 27). On the whole, dairy cattle feed should be kept away from contamination as much as possible. However, if the reduction of animal feed contamination is not practical, the use of highly contaminated feed should be diverted to non-lactating animals. According to our findings, AFM1 contamination of milk is not a major concern in this city. Of course, our investigation was a cross-sectional study performed in 2012. Therefore, the present situation may be varied. However, according to the climatic fluctuations, dairy cattle feed samples of various livestocks should be checked regularly for aflatoxin and the storage conditions of feeds must be strictly controlled.
Comparison of the Prevalence of Milk Contamination in Cities of Iran