1. Background
Tonsillectomy is one of the most common pediatric surgeries and followed by a severe and significant postoperative pain and leading to a negative impact on patient health and possibly causing dysphagia, bleeding and dehydration. Despite emphasis on pain control after tonsillectomy, about 50% of children undergone the procedure experience severe postoperative pain (1).
Post-tonsillectomy pain is usually treated by narcotic drugs that can raise the risk of breathing problems after surgery. Dexamethasone and other steroids are associated with some adverse effects such as adrenal dysfunction (2).
Bupivacaine is an anesthetic drug with trade name of marcaine. It belongs to amino amide drug group and has a hepatic metabolism. The drug half-life is 8 hours in children with effect duration of more than 24 hours. Its mechanism of action is through specific blockage of sodium and potassium channels at the end of nerve fibers and thus preventing the transmission of pain signals. The maximum allowable dosage of drug is 2 mg per kilogram of body weight. No certain adverse effects have been reported for the drug so far (3).
2. Objectives
This study aimed to investigate the effectiveness of topical bupivacaine on relief of post-tonsillectomy pain in children referred to Imam Khomeini Hospital of Ahvaz.
3. Patients and Methods
The study was a randomize double blind clinical trial (Registration ID: 2014041617296 N2) performed in Imam Khomeini Hospital, Ahvaz, in 2013 on 60 elective tonsillectomy patients. All patients signed a consent form. The patients were randomly divided into two groups of A and B. Both groups received the same anesthesia. Immediately after induction, patients in Group A received 0.5 mg/kg local injection of bupivacaine into the tonsillar bed, while the same amount of placebo (sterile water) was injected to patients in Group B. The type of procedure was cold knife and used suturing for control of bleeding. The face pain rating scale (FPRS) method, trained to parents and children before operation, was used to assess pain. There are six faces in this method from smiling to crying; patients are asked about their resemblance to the faces after surgery and the amount of pain is scored according to these faces. The test was performed at 6 and 24 hours after the operation and the results were recorded on data collection forms. The time to onset oral fluids and the time of the first analgesic request were recorded on data collection form. The study was continued until the completion of samples. Inclusion criteria included children who were supposed to undergo tonsillectomy (obstruction and infection indications) and parental consent. Exclusion criteria included a history of any drug allergy and lack of parental consent. This study was approved by the ethics committee. The results were statistically analyzed using SPSS-19.
4. Results
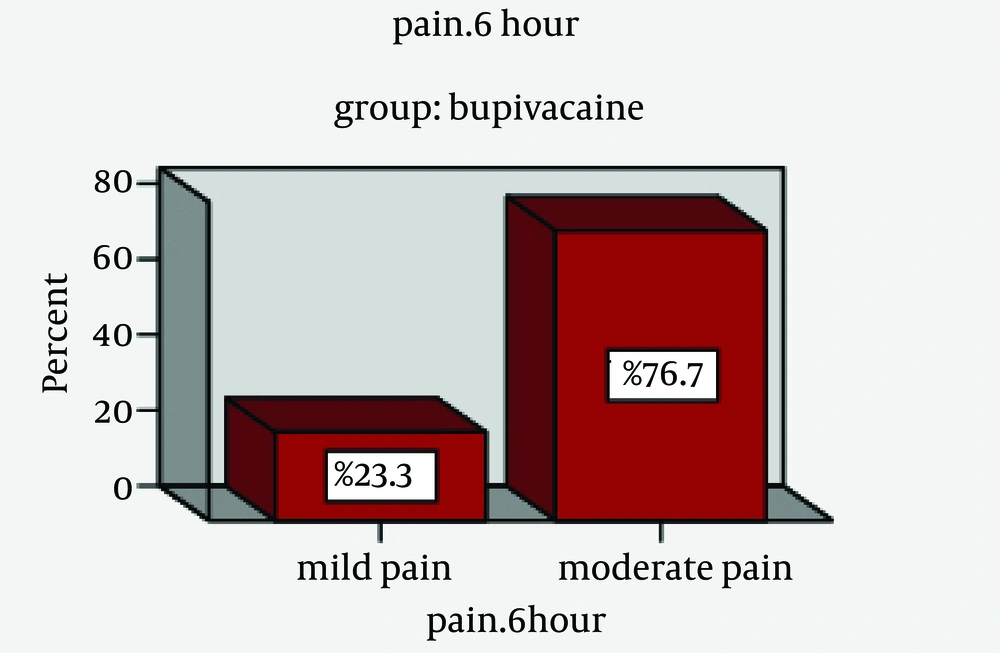
In this study, 60 patients with an age range of 4 - 13 years were studied in two groups of A (bupivacaine) and B (placebo) (Figure 1); of them 37 were male (61.7%) and 23 female (38.3%). Of 30 patients in group A (bupivacaine), 20 were male (66.7%) and 10 female (33.3%) and of 30 patients in Group B (placebo), 17 were male (56.7%) and 13 female (43.3%) (Table 1). In Group A, 27 patients (90%) did not request postoperative analgesic; this was 20 patients (66.7%) in Group B. Mean of request time in Group A (bupivacaine) was lower than Group B (P = 0.002). The mean time of fluid intake after the operation in Group A (bupivacaine) was lower than Group B (placebo) (P < 0.001). Mean of pain score six hours after the operation in Group A (bupivacaine) was significantly lower than the placebo group (P < 0.001). Mean of pain score at 24 hours after the operation was lower in the drug group (bupivacaine) than the placebo group (P < 0.001).
| Group | Age | Weight | First Time Request, h | Time Liquid, h | Pain Score, 6h | Pain Score, 24 h |
|---|---|---|---|---|---|---|
| Bupivacaine | ||||||
| N | ||||||
| Valid | 30 | 30 | 30 | 30 | 30 | 30 |
| Missing | 0 | 0 | 0 | 0 | 0 | 0 |
| Mean | 7.43 | 32.23 | 17 | 5.33 | 4 | 1.20 |
| Median | 7 | 30 | 0 | 5.00 | 4 | 1 |
| Mode | 6 | 26 | 0 | 5 | 4 | 0 |
| Std. deviation | 2.112 | 8.152 | 0.531 | 0.758 | 1.390 | 1.349 |
| Minimum | 4 | 20 | 0 | 4 | 2 | 0 |
| Maximum | 13 | 48 | 2 | 7 | 6 | 4 |
| Placebo | ||||||
| N | ||||||
| Valid | 30 | 30 | 30 | 30 | 30 | 30 |
| Missing | 0 | 0 | 0 | 0 | 0 | 0 |
| Mean | 7.67 | 34.83 | 0.73 | 6.30 | 7.80 | 4.60 |
| Median | 7 | 34.50 | 0 | 6 | 8 | 4 |
| Mode | 5 | 32 | 0 | 6 | 8 | 4 a |
| Std. deviation | 2.309 | 8.987 | 1.202 | 0.915 | 1.609 | 1.754 |
| Minimum | 5 | 16 | 0 | 5 | 4 | 2 |
| Maximum | 13 | 48 | 4 | 8 | 10 | 8 |
a Multiple mode exist, the smallest value is shown.
5. Discussion
As one of the most common pediatric surgeries, tonsillectomy has a severe and significant postoperative pain, which may cause dysphagia and dehydration in patients. Different methods can be used to reduce pain (1, 4). Opioid analgesics are associated with the risk of respiratory depression; dexamethasone and other steroids can suppress the adrenal gland. Since no serious adverse effect is reported for bupivacaine and given its high half-life, it can be used as a safe drug to reduce post-tonsillectomy pain. In 2012 in Lahore, Hashmi et al. studied the impact of bupivacaine injection on pain and nausea after tonsillectomy. The group that received bupivacaine clearly had less pain and nausea (P < 0.001) (3). In the present study, the mean score of pain was clearly lower in the bupivacaine group at 6 and 24 hours after the operation (P < 0.001). In a study in Turkey, Tekelioglu evaluated three groups of 20 persons (group A with bupivacaine, group B with dexamethasone and group C with placebo); the group that received bupivacaine had less pain (5) and the results were similar to those of the present study (P < 0.001). In another study by Ozmen in Turkey (6), three groups received local injection of bupivacaine, lidocaine and normal saline; the same as the present study, the pain in the bupivacaine group was clearly lower (P < 0.001). Given that tonsillectomy is one of the most common surgeries in children and since patients have a great pain after the operation, it seems reasonable to use a safe drug such as bupivacaine as a treatment protocol to reduce pain and shorten return of children to normal life and community.