1. Background
Myocardial infarction (MI) is an important coronary heart disease (CHD). Twenty minutes after the onset of coronary occlusion, irreversible myocardial ischemic injury is begun in the subendocardium and progresses to the subepicardium of the ischemic myocardial bed at risk, and then completed after 3 to 4 h or less. In this situation, the infarcted cells showed signs of necrosis and death (1). Intracellular calcium and reactive oxygen species (ROS) are increased by myocardial cell injury due to ischemia/reperfusion (I/R), then the sarcolemmal lipid peroxidation leads to the loss of cellular integrity and facility of calcium entry. Therefore, pretreatment with antioxidant compounds can protect cells against ROS injuries.
In recent studies, scientists have found that vegetables and fruits are rich sources of antioxidants, which can defend the heart against CHD (2). In addition, they proved that Gallic acid and other antioxidants (3) play a protective role (related to their phenolic compounds) against oxidative stress (4). Calcium is entered in the mitochondria because of ROS generation, during reperfusion (5). In intact cells, high Ca2+ levels can be supplied from ryanodine release receptors (RyRs) and 3-phosphatidylinositol receptors (IP3R). Another pathway is calcium entry into the cardiomyocytes through the voltage-dependent Ca2+ channel (VDCC) (5). Also the opening of the mitochondrial permeability transition pore (mPTP) with its accessory components such as cyclophilin D (CpD), and cardiolipin (CL) is modulated by calcium (6).
Thus, preservation of mitochondria against oxidative stress can prevent and reduce the cellular damages which are caused by ROS. Also, Priscilla et al. showed that pretreatment with Gallic acid reduced the necrosis in the histopathological finding of infarcted cardiac muscle morphology in male Wistar rats (4). Furthermore, another study has reported that apricot-feeding has cardioprotective effects on necrosis and histopathological findings during I/R in rat. The beneficial effects can be attributed to its phenolic compounds (3). The effects of different doses of Gallic acid on histopathological changes during I/R on myocardial morphology have been investigated, but there is no research on either COL fibers or mitochondrial protection. It has been proved that CsA increases dose-dependently ROS synthesis at 1 µM (7), so we chose the lower dose of CsA that was not cytotoxic.
2. Objectives
Considering all the above-mentioned issues, we investigated the dual cell and mitochondria membrane protection and their combined effects of Gallic acid (as an antioxidant) and CsA (as mPTP inhibitor, to prevent opening mitochondria pores) on myocardial morphology and COL during I/R in rats.
3. Materials and Methods
3.1. Animals
Fifty-four male Wistar rats (250-300 g) obtained from the Animal House of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran, were randomly divided into 9 groups (Table 1). The sham group received neither Gallic acid nor CsA. In the control group, animals were exposed to 30 minutes ischemia following 60 minutes reperfusion. Control group was divided into 2 subgroups: Ca group received saline as the solvent of Gallic acid and Cb group was pretreated by saline and then perfused with CsA. The Gallic acid groups were pretreated with different doses of Gallic acid in saline (G1a:7.5, G2a:15, and G3a: 30 mg/kg/day). Gallic acid was dissolved in saline and administered to animals using gavage tube for 10 days (8). The other groups pretreated both with different doses of Gallic acid and perfused with CsA during the first 13 minutes of reperfusion (G1b:7.5, G2b:15 andG3b: 30 mg/kg/day). All the animals in these groups were ischemic except sham group. Animals were maintained under the same conditions such as controlled room temperature (22 ± 2°C), with a 12 hours dark-light cycle supplied with food and water ad-libitum. This study was approved by the animal care and Ethics Committee of the Ahvaz Jundishapur University of Medical Sciences. Also this study was a quality research, which was done in the Physiology Research Center of the Ahvaz Jundishapur University of Medical Sciences during 2012.
3.2. Chemicals
Gallic acid, cyclosporine A (CsA), heparin, hematoxylin and eosin, and Masson’s trichrome alcohol, were purchased from Sigma (St. Louis, MO USA); sodium chloride, potassium chloride, magnesium sulfate, sodium hydrogen carbonate, potassium hydrogen phosphate, D-glucose and calcium chloride were obtained from Merck Laboratories. Ketamine and xylazine were bought from Alfasan Co (Holland).
With regard to preparation and isolation of heart perfusion, the animals were anesthetized by ketamine HCL (50 mg/kg), and xylazine (5 mg/kg) which contained heparin (1000 U/kg, IP). The trachea was cannulated, and the animals were ventilated with room air using a rodent ventilator (UGO- BASILE, model: 7025) (9). Chest was opened and the ribs removed, then a steel cannula was placed into the aorta and secured with a suture. The heart was immediately perfused with Krebs-Henseleit bicarbonate solution (bubbled with 95% O2 and 5% CO2 to attain a pH of 7.4). Then, the heart was quickly excised and transferred to a Langendorff apparatus while continuously perfused with Krebs-Henseleit solution at a constant pressure (60-70 mm Hg) at 37°C. All hearts were perfused for 30 minutes before the induction of no flow global ischemia (30 minutes) to allow hemodynamic equilibration, followed by 60 minutes of reperfusion (10).
Ischemia was induced by cutting the Krebs-Henseleit flow using the pressure-flow controller key (off) for 30 minutes. It was determined by ST elevation on the electrocardiogram. Heart rate and perfusion pressure were monitored continuously (9). CsA (0.2 µM) was dissolved in ethanol and added to Krebs-Henseleit solution 13 minutes before the induction of ischemia and continued during reperfusion (11, 12). After the required period of I/R, the heart was removed from the cannula, then preserved in 10% formalin for histological studies.
3.3. Sample Staining with Hematoxylin and Eosin
Small pieces of the heart were fixed in 10% formalin and processed using a standard histological procedure. Sections of 6-µm thickness were stained with hematoxylin and eosin. Then, the tissue sections were evaluated for histological changes under Olympus BX51 light microscope. In another step, 6-µm sections were stained with Masson’s trichrome staining to consider changes in COL fiber network (11). The intensity of the damage was checked by scaling in three levels (severe, moderate and mild) (Table 2).
| Animal group | Edema | Contraction Band | Pyknotic Nuclei | Inflammatory Cells | Collagen Fibers | Grade |
|---|---|---|---|---|---|---|
| Sham | - | - | - | - | + | - |
| Control | severe | - | a lot of | a lot of | - | III |
| CsA | severe | Some | some | rare | diffusely | II |
| G7.5 | moderate | - | some | some | focally | III |
| G7.5 + CsA | severe | - | some | few | focally | II |
| G15 | moderate | Some | few | - | diffusely | II |
| G15+ CsA | mild | A lot of | - | - | diffusely | І |
| G30 | severe | few | a lot of | few | - | III |
| G30+ CsA | severe | few | a lot of | few | - | III |
Comparison of Heart tissue Damage Intensity in Different Groups in Normal and Myocardial Infarcted Rats (n = 6)a
3.4. Statistics
Comparison between different groups was assessed using Pearson’s chi-squared test, and data were presented as mean ± SD (SPSS 18 software). P values below 0.05 were considered as statistically significant.
4. Results
4.1. Sample Staining With Hematoxylin and Eosin
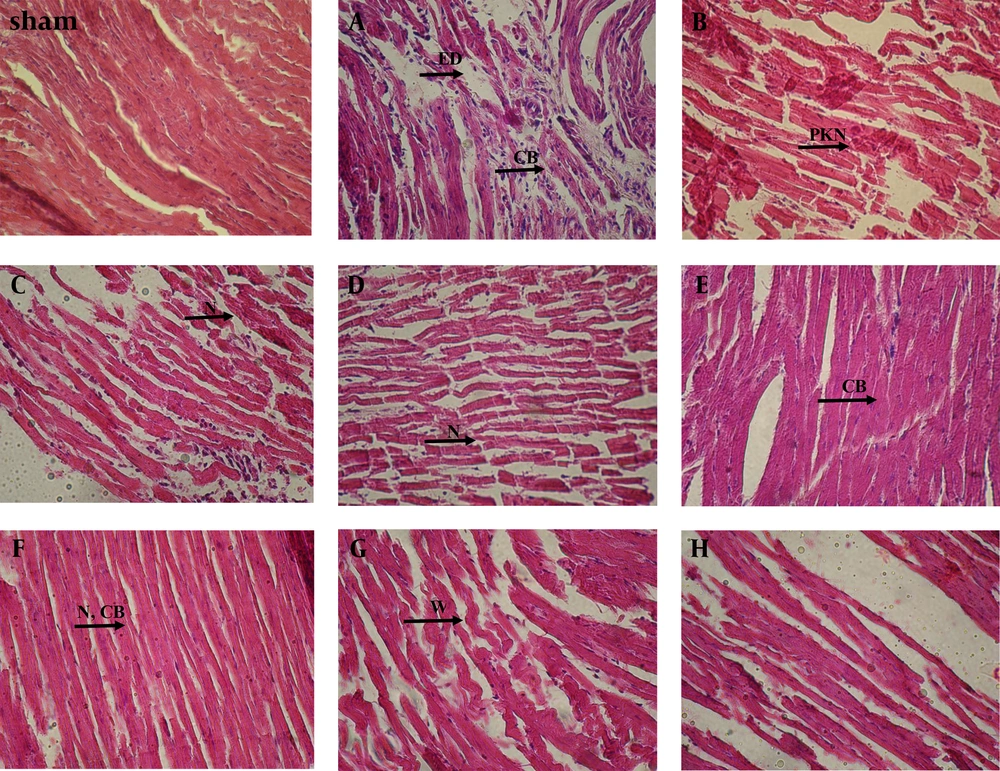
Tissue from the control (Ca) rats showed severe edema (ED) between muscle fibers, infiltration of many inflammatory cells (IC), pyknotic nuclei (PKN), and absence of contraction band (CB) (Figure 1A) more than either sham or other groups, which were perfused with CsA alone (Cb) (Figure 1B). Although some normal muscle fibers, IC and the presence of rare contraction bands had shown in the latter group (Cb) (Figure 1B). Pretreatment with Gallic acid (7.5 mg/kg) (Figure 1C) showed severe ED, IC and necrosis (N) between muscle fibers more than the cases which received Gallic acid (7.5 mg/kg) in combination with CsA (Figure 1D). Also, we observed fewer normal cells with normal nuclei in the second group (G7.5 + CsA) rather than ones, which perfused with CsA alone.
Pretreatment with Gallic acid (15 mg/kg) and reperfusion with CsA (Figure 1F) showed mild edema, normal architecture of myocardium with a lot of contraction bands (CB), and oviform nuclei more than which exposed to Gallic acid (15 mg/kg) or CsA alone (Figure 1E). However, a few necrotic cells with pyknotic nuclei and moderate edema were observed in the latter groups (G15, Cb). Pretreatment with Gallic acid (30 mg/kg) (Figure 1G) and combination of Gallic acid (30 mg/kg) and CsA (Figure 1H) showed severe ED, PKN and many wavy fibers (W). In both groups, the normal cells were rarely observed (Table 2).
Edema (ED), pyknotic nuclei (PKN), contraction bands (CB), necrosis (N), wavy fibers (W). Sham: a group that did not expose to ischemia. A, control group received saline; B, group received CsA; C, group received Gallic acid (7.5 mg/kg); D, group received Gallic acid (7.5 mg/kg + CsA); E, group received Gallic acid (15 mg/kg); F, group received Gallic acid (15 mg/kg + CsA); G, group received Gallic acid (30 mg/kg); H, group received Gallic acid (30 mg/kg + CsA).
4.2. Sample Staining With Masson’s Trichrome
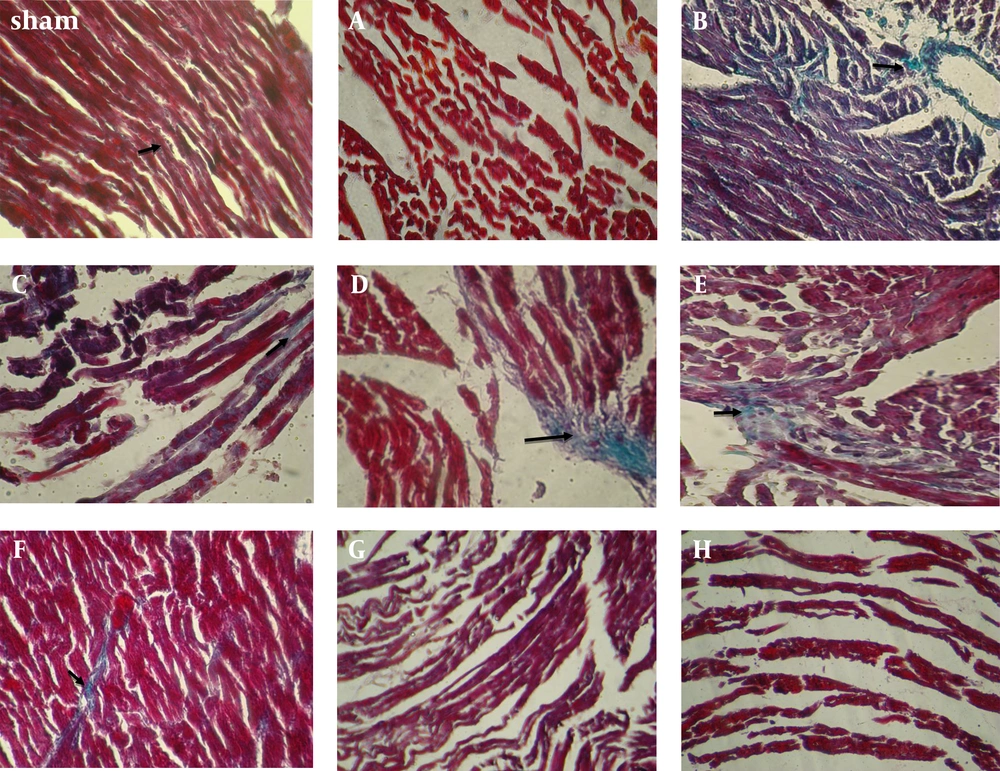
Tissue from control rats showed loss of myocardial structure without collagen fibers that represent severe edema between the myocardial cells, so woven myocardial structure was observed in this group that had undergone 30 minutes ischemia and 60 minutes reperfusion (Figure 2A). Perfused tissue with CsA showed a presentation of connective tissue containing diffused but woven collagen (COL) fibers between some of the normal myocardial cells (Figure 2B). Pretreatment with Gallic acid (7.5 mg/kg) showed a few thin COL fibers and necrosis in the majority of the connective tissue cells, compared with the ones received Gallic acid (7.5 mg/kg) and CsA (Figure 2 C); therefore, general muscle structure has gone wavy mode.
Pretreatment with Gallic acid (7.5 mg/kg) and CsA showed some focally and thin COL fibers more than those which received CsA or Gallic acid (7.5 mg/kg) alone (Figure 2D). Pretreatment with Gallic acid (15 mg/kg) and CsA showed a normal connective tissue with diffused thick COL fibers between the most cells in comparison with the ones perfused with CsA alone (Figure 2E and F). Pretreatment with Gallic acid (30 mg/kg) (Figure 2G) and combination of Gallic acid (30 mg/kg) and CsA (Figure 2H) showed the extensive COL damage and loss of normal myocardial architecture with marked waviness (W) in the most cells in comparison with groups which perfused with CsA alone. So the groups of cells were appeared (Table 2).
Sham: a group that did not expose to ischemia, A, Control group received saline; B, group received CsA; C, group received Gallic acid (7.5 mg/kg); D, group received Gallic acid (7.5 mg/kg + CsA); E, group received Gallic acid (15 mg/kg); F, group received Gallic acid (15 mg/kg + CsA); G, group received Gallic acid (30 mg/kg); H, group received Gallic acid (30 mg/kg + CsA).
5. Discussion
During MI, activation of anaerobic glycolysis to provide ATP leads to accumulation of H+ and acidosis. In response to elevated levels of H+, Ca2+ uptake into mitochondria occurred by the mitochondrial Ca2+ uniporter. These changes regenerate the required ion gradient for more Ca2+ entry into the mitochondria, which causes the long-lasting opening of mPTP regulated by CypD and mitochondrial swelling leads ultimately to cellular necrosis (13). In accordance with two light microscopy studies, we found some changes between muscle fibers such as edema, degeneration, pyknotic nuclei, wavy contraction bands and loss of the COL network after 30 minutes ischemia followed by 60 minutes reperfusion.
The results of this study, have shown that pretreatment of the animals with Gallic acid as an antioxidant or CsA as mPTP inhibitor alone, somewhat reduced mentioned changes; however, a combination of both drugs improved the morphology of cells more than each one of them alone. Another study has shown that during acute ischemic phase, matrix metaloproteinas (MMP) activity increases due to proteolytic and redox stress. It could be followed by degradation of the extracellular matrix (ECM) at the ischemic region, thus tissue inhibitor of metaloproteinases (TIMPs) concentration is lowered and the components of ECM (like collagen) are degraded. Structural remodeling of the infarcted myocardium leads to tissue stretching, hypertrophy and fibrosis (14). Collagen has an important role in keeping myofibers together concomitant with myofibrillar protein. Thus, I/R injury can affect the myocardial function after losing of COL and myocardial performance change by MMPs in the early stage of cell damage (10).
During ischemia, collagenolytic activity was increased and followed by decrease in MMP1 activity (10). Balance between TIMP inhibition and collagenase activation determines degradation of interstitial matrix and the amount of collagenolysis (14). In addition, other enzymes like lysosomal proteases (such as cathepsin G) are responsible in this process in infarcted tissues (14). Likewise, changes in the mitochondrial morphology of cardiac myocytes have been shown in other related studies (15). Furthermore, Hausenloy et al. showed that inhibition of mPTP opening reduced both necrotic and apoptotic cells and preserved mitochondrial morphology (16); thus, this effect prevents I/R injury. Previous researchers showed that Gallic acid preserved the integrity of lysosomal membrane and reduced the level of serum troponin (a contractile protein) by keeping the activities of different fraction of lysosomal enzymes in isoproterenol induced cardiotoxicity in rat heart tissue (4, 8). Also it was shown that ROS leads to cell are organelle membrane lipid peroxidation and mitochondrial enzyme dysfunction.
As a result of these events, the mitochondrial membrane is depolarized, and mPTP opened. Then because of anaerobic metabolism, ROS production increases and exacerbates the oxidative stress (5, 17) to make a vicious cycle. Therefore, Hypothesis of this research was to assess the combination effects of CsA (a potent inhibitor of mPTP) with Gallic acid (a potent antioxidant) on myocytes necrosis and morphology during I/R injury. The results of this study, have shown that pretreatment of the animals with Gallic acid or CsA alone would reduce edema, regenerative changes and COL damage, but a combination would improve the morphology of cells and keeping COL fibers more than each one alone.
In addition, Loss of Col and waviness of the myocytes of the control group in the current study, could be attributed to MMPs activity (14) or lysosomal enzymes like cathepsin D and L (18) that previously mentioned by Tiwari et al. (19). The preservation of COL and myocyte morphology in treatment groups, may be due to the preservation of mitochondria against oxidative stress with CsA as well as the elevation of antioxidant capacity with Gallic acid which prevents damage to cell mitochondrial and lysosomal membranes against lipid peroxidation. Therefore, co-administration of both CsA and Gallic acid appeared more effective. Because, we protected the cell with two mechanisms, it may further prevent damage caused by oxidative stress and the above-mentioned vicious cycle. we observed that collagen had been preserved and regenerative changes decreased remarkably in the heart tissue sections which pretreated by Gallic acid at the medium dose (15 mg/kg), compared with the control group (20).
Similar to Priscilla (4), we did not find a significant positive effect at the lowest dose of Gallic acid (7.5 mg/kg). But using the highest dose (30 mg/kg) reversed these effects. According to another study, it can be explained by a pro-oxidant effect of Gallic acid in high doses, because it has proven that ferric ion can chelate the hydroxyl group in Gallic acid molecule and reduces the oxidation potential so it losses its antioxidant activity (21). On the other hand, CsA increases the ROS synthesis in doses higher than 1 µM (7); thus, we used safety dose which does not have toxic effects (0.2 µM) (12). Also, we observed that whenever the mitochondria was protected, the tissue damage due to I/R injury would be more reduced. Hence, this result can be useful to protect the heart after ischemia and at the onset of reperfusion.
In conclusion, the present results approved our hypothesis that the combination of CsA and Gallic acid preserved the myocardial cell morphology against I/R injuries, which was more effective than using either of them alone. This effect has the best activity in the medium dose of Gallic acid (15 mg/kg) with CsA.