1. Background
Dandruff is characterized as a hyperproliferation of the scalp epidermis accompanied with scalp itching and redness (1). Dandruff mechanism is thought to be the result of the activity of enzyme called lipase (2). The Malassezia fungus (cause dandruff) uses this enzyme to break down sebum to oleic acid (pro-inflammatory free fatty acids) (2). Also, this fatty acid penetrates into the top layer of scalp and causes inflammation and increased skin cell flaking, in susceptible people (2, 3). Nowadays many chemical treatments are available for reducing and removing highly resistant dandruff against therapeutic cure, on the other hand, there are many antidandruff productions with poor clinical efficacy in markets (4). Due to these concerns, now days, a great tendency to use medicinal plant extracts is revealed to treat dandruff. Some studies showed that extracts of Punica granatum L. (5, 6), Rosmarinus officinalis L. (7, 8), Matricaria chamomilla L. (9), Urtica dioica L. (10), Mentha piperita L. (11, 12), and Salvia officinalis L.(12) remove scalp dandruff, or at least decrease it to high extent. The aim of this study was to investigate the clinical trial for dandruff therapy by Zinc L-pyrrolidone carboxylic acid (Zinc-PCA) and pirocton olamine in combination with the above-mentioned six medicinal plant extracts.
Punica granatum L. belongs to the family Punicaceae (13). This plant has been used for thousands of years to cure a wide range of diseases, especially dandruff and scalp inflammation. It is one of the most important endemic plants in Iran (14).
Punica granatum L. chemical constituents are ellagic acid, ellagitannins (including punicalagins), luteolin, β-sitosterol, icosanoic, linolenic, citric acid, malic acid, protocatechuic acid, chlorogenic acid, caffeic acid, ferulic acid, coumaric acid, anthocyanins, polyphenols, flavonoids and tannins (15-17). In various studies reported that these chemical compounds have various pharmacological properties, including anti-itching, antidandruff, anti-inflammatory (by inhibiting pro-inflammatory cytokines) and antioxidant (18-22). They also have inhibitory effects on enzymes cyclooxygenase (COX), lipooxygenase (LOX) and Phospholipase A2 (PLA2) (23, 24). As COX and LOX are key enzymes in converting arachidonic acid to prostaglandins and leukotriene’s which cause inflammation (25, 26). Also tannin, ellagic acid and phenolic acid in the plant possess antidandruff, antifungal and antimicrobial properties (5, 6).
Rosmarinus officinalis L. is native of Mediterranean and related to the family Lamiaceae. This plant grows widely in large areas of southern Europe, northern Africa, England, Mexico and USA. Rosmarinus officinalis chemical compounds include 1,8-cineole (15 - 55%), β and α-pinene (9 - 26%), camphor (5 - 31%), resin, tannic acid, flavonoids, volatile oils consists of borneol (1.5 - 5%), lomonene (1.5 - 5%), comphene (2.5 - 12%) and cineole (27, 28).
This plant has been traditionally used as an antibacterial and antifungal effective ingredient due to reduce fatty acid peroxidation and inhibit fungi growth in pH = 5 - 6 by membrane damage, related to the loss of ions and membrane potential reduction, collapsing proton pump and ATP depletion (7, 29) Moreover, the antioxidant properties of the R. officinalis methanolic extract is attributed to its phenolic compounds, flavonoids, rosmarinic acid, natural pigments such as capsaicin and curcumin, and terpens such as carnosic acid and carnosol (8, 30).
Matricaria chamomilla L. is an annual plant related to the family Asteraceae and it mainly grows in Europe, Asia and Africa (31). The main constituents of this plant include terpenoids α-bisabolol (56%), luteolin, chamazulene and azulenes that they have properties anti-inflammations by blocking cyclooxygenase enzyme in the synthesis of prostaglandins and inhibit leukotriene formation. Matricaria chamomilla L. components also relieve skin and inhibit fungal growth (32, 33). The other compounds of M. chamomilla are caffeic acid, flavones: apigenin, glycoside, flavonols: quercelin, rutin, uronic acid and a bitter substance named anthemique acid acts as a great anti-allergic substance. Moreover, studies have shown that the mentioned herb inhibits prostaglandin formation, cyclooxygenase: (COX-1), (COX-2) and central enzymes in pro-inflammatory pathways. As well as it blocks histamine release, based on this fact, its anti-inflammatory effect is revealed (9, 34).
Urtica dioica L. is a perennial plant and belongs to the family Urticaceae. It grows in temperate regions such as Asia, Europe and America. This plant essential oils and extract contains formic acid and acetic acid, histamine, tannins, mucilage, vitamins (A, B1, B2 and C), lutein and lycopene have medicinal effects. Interestingly, U. dioica inhibits the 5-α-reductas enzyme and promotes blood flow to nourish follicles due to β-sitosterol and ursolic acid (10).
Mentha piperita L. is a perennial and important therapeutic plant that belongs to the family Labiate. The plant is endemic of Europe, although now it is being cultivated all around the world. M. piperita leaves contains 0.5 - 4% volatile oil (50 - 78% free menthol, menthone, monoterpene and menthofurane) (11, 12, 35). This oil has a notable role in alleviating pain and improving blood flow in the scalp (36, 37). Azulenes, carotenes, coline, essential oil (containing β and α-pinene), cineole, limonene (1 - 5%), flavonoids: menthoside (14 - 32%), rosmarinic acid and tannins are inhibitory to bacteria, fungi and yeasts growth (38, 39).
Salvia officinalis L. is medicinal and aromatic plant belongs to the family Lamiaceae, which is native to Mediterranean countries (39). An important chemical compounds of this plant include linoleic acid, gallic acid, ursolic acid, luteolin and apigenin for removing dandruff and fungus (39, 40). Additionally, rosmarinic acid, caffeic acid, gallic acid, flavonoids, phenolic acid, caumarins, tannins (41-43), 1 and 8-cineole, campher and borneol, possess strong anti-inflammatory, antifungal and antibacterial activities (40, 44) by inhibiting liposomes lipid peroxidation (45). These biological effects act through modulation of signal transduction pathways, inhibiting cyclooxygenases and lipoxygenases (46, 47). In this study, antidandruff and anti-inflammatory properties of the mentioned six medicinal plants in combination with two chemical compounds pirocton olamin (PO) and Zinc PCA are being examined on hair with dandruff, in order to evaluate the efficacy of extracts on dandruff removal and reducing chemical side effects.
2. Objectives
The aim of this study was to investigate the clinical trial for dandruff therapy by zinc L-pyrrolidone carboxylic acid (Zinc-PCA) and pirocton olamine in combination with six medicinal plant extracts.
3. Patients and Methods
3.1. Plant Preparation
For this study, flowers of Punica granatum L. (Markazi Province, gardens in Saveh City), Rosmarinus officinalis L. leaves (Mountains near Karaj), Matricaria chamomilla L flowers (Damavand Province), Urtica dioica L. roots (Shemiranat Province), Mentha piperita L. leaves (forest in northern of Alborz province) and Salvia officinalis L. leaves (heights of Mashhad) were collected between June 2011 and July 2011 and recognized by Herbarium expert of the Pars Azmaye Teb (Cerita) company, based on Flora Iranica. Table 1 shows address of the area of collected plants for the extraction of plant.
| Name of Plant | Locations of the Plant Collection | Address of Plant Collection | |
|---|---|---|---|
| Latitude | Longitude | ||
| Punica granatum L. | Markazi Province, gardens in Saveh City | N 35° 1’ 17.9” | E 50° 21’ 15.4” |
| Rosmarinus officinalisL. | Mountains near Karaj | N 33° 30’ 22” | E 52° 27’ 0” |
| Matricaria chamomilla L. | Damavand Province | N 32° 31’ 41” | E 51° 30’ 0” |
| Urtica dioica L. | Shemiranat Province | N 35° 57’ 51.7” | E 51° 34’ 54.4’’ |
| Mentha piperita L. | Forest in northern Alborz province | N 35° 40’ 57.0” | E 51° 24’ 44.4” |
| Salvia officinalisL. | Heights of Mashhad | N 37° 4’ 29” | E 59° 4’ 47” |
3.2. The Plant Extraction Method
Plants were carefully and attentively collected from mentioned places and dried in sheets within a week in clean, dry and dark room. Flowers of P. granatum, M. chamomilla and S. officinalis, leaves of M. piperita and R. officinalis and U. dioica roots were fully separated and milled. In this stud, Soxhlet apparatus was used for extraction and according to Tekli et al. method also Rotary evaporation apparatus was used to evaporate methanol (48). Then 15 g of each powder was dissolved in 300 mL methanol 96% (Merck, Germany) and shaked for 24 hours by shaker (KS 130 Control Mixing Orbital Shaker, IKA Company, Germany) after necessary time shaking, these solvents were filtered by Whatman NO. 1 and Rotary evaporation (RV 10 Digital Rotary Evaporator IKA Company, Germany) in 90 rpm, 50°C and 15 minutes was used to evaporate methanol, up to 10 mL for preservation in vials in 4°C. For producing pure extracts, methanolic solvents in vials remain under hood for 24 hours till full evaporating and based on musilagic form of extract, DMSO was used to dissolve it and combine it with chemicals.
3.3. Physicochemical Tests
In the present study, the effects of the 6 medicinal plants on dandruff treatment were investigated. Then the physic-chemical parameters such as moisture content, pH (1% aqueous), total ash, acid insoluble ash, alcohol and water soluble extractives, and preliminary phytochemical screening for the presence of alkaloid, flavonoid, glycoside, phenol, saponin and tannin were studied using the standard procedures as follows (49):
Moisture content: 5 g of powdered material weighed accurately in a dry and flat petri dish and the sample was dried in an oven at 110°C (drying was carried out for two days and the loss of weight was calculated in terms of percentage).
pH (1% aqueous): 1 g of powdered sample was dissolved in 100 mL of distilled water; then was stood for 18 hours and sample was filtered and the pH was checked using a pH meter.
Total ash: about 2 - 5 g of dried plant material weighed accurately in a previously ignited and tared crucible. Then it was gradually ignited by heating in 500°C - 600°C until it to be white. It was cooled in a desiccator, and weighed and finally the content of total ash was calculated in terms of percentage.
Acid insoluble ash: Twenty-five mL of Hydrogen chloride HCl (~ 70gr/mL) was added to the crucible containing total ash, and was covered with watch glass then boiled gently for 5 minutes. The watch glass was rinsed with 5 mL of hot water and this liquid was added to the crucible. The insoluble matter was collected on an ashless filter paper and washed with hot water. The filter paper containing insoluble matter was ignited in the crucible to constant weight then was cooled in a desiccator. It was weighed and the content of acid insoluble ash calculated in terms of percentage.
Alcohol soluble extractives : Approximately 4 g of air-dried material was weighed accurately in a glass stoppered conical flask and macerated with 100 mL of absolute alcohol for 6 hours (with shaking frequently) then stood for 18 hours. Solution matter was filtered rapidly taking care not to lose any solvent and 25 mL were transferred to a tared flat bottomed petri dish and evaporated to dryness on a water bath and at 105°C for 6 hours. Finally, it was cooled in a desiccator for 30 minutes and weighed and the content of alcohol-soluble matter was calculated in terms of percentage.
Alkaloid (Wagner’s test): Acidify 1 mL of alcoholic extract of the plants with 1.5% of HCl and add a few drops of Wagner’s reagent (a brown precipitate indicates positive test for alkaloids).
Flavonoid: In a test tube containing 0.5 mL of the alcoholic extract of plants, add 5 - 10 drops of dilute HCl, followed by a small piece of magnesium. Boil the solution for a few minutes (in the presence of flavonoids a pink, reddish pink or brown color is produced).
Glycoside: Dissolve a small amount of alcoholic extract of plants in 1 mL of water and add 1 Normal (N) Sodium hydroxide (NaOH) solution (a yellow color indicates the presence of glycosides).
Phenols (FeCl3 test): Dissolve a small quantity of alcoholic extract of plants in 2 mL of distilled water and a few drops of 10% ferric chloride solution (a blue or green color is produced indicates the presence of phenols).
Saponins: Dissolve a small quantity of alcoholic extract of plants in 5 mL of distilled water, shake the mixture vigorously and leave for 3 minutes (honeycomb like froth indicates the presence of saponins).
Resin: Dissolve a small quantity of the methanolic extract of plants in 5 mL of acetic anhydride by means of gentle heat, cool and add a drop of sulphuric acid (a bright purplish red color indicates the presence of resins).
Tannin: To prepare the methanolic extract of plants, add a few drops of 5% aqueous ferric chloride solution (a bluish black color indicates the presence of tannins).
The slight variation in physicochemical and phytochemical results may be due to several factors such as different geographical conditions, edaphic factors, environmental conditions, period of cultivation and harvesting, method of collection, source of irrigation and fertilizers, age of the plant, powdering method, and extraction method. Table 2 and 3 show the results of the physico-chemical study.
| Name of plant | Moisture Content | PH (1% Aqueous) | Total Ash | Acid Insoluble Ash | Water-Soluble Extractive | Alcohol Soluble Extractive |
|---|---|---|---|---|---|---|
| Punica granatum L. | 7.04 | 4.5 | 3.55 | 0.13 | 30.1.1 | 20.4 |
| Rosmarinus officinalis L. | 8.3 | 5.6 | 4.10 | 0.21 | 23.4 | 10.7 |
| Matricaria chamomillaL. | 10 | 6.7 | 3.2 | 0.44 | 30.1 | 12 |
| Urtica dioica L. | 9.3 | 6.31 | 5.1 | 0.26 | 21.8 | 9.25 |
| Mentha piperita L. | 11.1 | 6.98 | 7.2 | 0.36 | 22 | 15.25 |
| Salvia officinalis L. | 10.4 | 6.92 | 2.32 | 0.15 | 23.56 | 14.6 |
aData are presented as gram.
| Name of plant | Alcohol Extract Yield | Aqueous Extract Yield |
|---|---|---|
| Punica granatum L. | 28.1 | 12.8 |
| Rosmarinus officinalisL. | 41 | 37.1 |
| Matricaria chamomilla L. | 32.7 | 25.5 |
| Urtica dioica L. | 31 | 29.6 |
| Mentha piperita L. | 34 | 23 |
| Salvia officinalisL. | 23.4 | 18.6 |
aPercent by weight-weight (%w/w).
3.4. Determination of Extraction Yield (% yield)
The yield (% w/w) from all the dried extracts was calculated as:
Where W1 is the weight of the extract after lyophilization of the solvent, and W2 is the weight of the plant powder. Table 4 shows the percentage extract yield of medicinal plants.
| Name of Plant | Alkaloid | Alkaloid | Glycoside | Phenol | Saponin | Resin | Steroid | Tannin |
|---|---|---|---|---|---|---|---|---|
| Punica granatum L. | + | - | + | + | + | - | + | + |
| Rosmarinus officinalisL. | + | + | + | + | + | + | - | + |
| Matricaria chamomilla L. | + | + | + | - | + | - | + | + |
| Urtica dioica L. | + | + | + | + | + | + | + | + |
| Mentha piperita L. | + | + | + | + | - | + | + | + |
| Salvia officinalisL. | + | + | - | + | + | - | - | + |
a(+), There active ingredient; (-), Absence of active ingredient.
3.5. Producing Shampoo With Necessary Materials for Assaying in Trial
A basic shampoo containing methanolic extract of P. granatum, R. officinalis, M. chamomilla, U. dioica, M. piperita, S. officinalis (each of them 0.1% V/V) were combined with sodium laureth sulfate 70% (10% V/V) and panthenol (0.3% V/V) and polyquaterium-10 (0.5 V/V) sigma aldrich, (USA) also salicylic acid (1% V/V) and disodium-ethylenediaminetetraacetic acid (EDTA) (0.3% V/V) and triethanolamine (0.2 V/V) and methychloro isothiazolinon ( 0.1 V/V) and butylene glycol (0.2 V/V) (Merck, Germany), cocamido propyl betaine 30% (7% V/V) and cocamide diethanolamine (DEA) 85% (1.2% V/V) (bought from Iranian Chemical Process Company), finally deionized water (100% V/V) (provided in Department of Pilot Nanobio Technology- Pasteur Institute of Iran), which were provided according to standards of shampoo in Iran. In the next step, PO 1% and zinc-PCA (Sigma Aldrich, USA) were added to the basic formulation for preparing new antidandruff shampoo (viscosity of about 8000 cp and pH = 5.5). The type of extracts, concentration and other ingredients in the shampoo is selected according to national and international standards (USP) and Institute of Standards and Industrial Research (FCC or CFR) in Iran.
All clinical trials were assessed based on terminology, and defined in the ICH E8 guidance (50) and safety pharmacology and pharmacodynamic (PD) studies have been defined ICH S7A (51). Also, for ethical approval of studies and human experimental investigations used from principles outlined in the Declaration of Helsinki (World Medical Association) (52, 53). The ethical principles are emphasized to select and participate voluntary patients and the responsibility of research subjects protection by physician or other heath care also is mentioned the primary propose of medical research for human subjects such as understanding the causes, development and effects of diseases and improving preventive, diagnostic and therapeutic interventions. Therefore, in this study, even the smallest interventions evaluated continually through research for patients’ safety, effectiveness, efficiency, accessibility and quality of treatment. Also, performance of each research study on patients described clearly in a research protocol (This protocol include personal information, incentives for treatment, assessment of predictable risks; improvement in treatment etc.). Finally, every precaution was taken to protect the privacy of research subjects and the confidentiality of their personal information and minimize the impact of the study on their physical, mental and social integrity.
3.6. Antidandruff Assay
Thirty patients (13 males and 17 females, ages 15 to 60 years) suffering from dandruff were randomly selected and asked to apply this combination in the form of PZ+ shampoo. Patient’s characteristics are given in Table 5. This experiment lasted 2 months by applying shampoo 3 times a week. Results were closely investigated to evaluate any symptoms of chemical side effects or any dandruff removal or reduction.
| Age, y | Number of Patients | |
|---|---|---|
| Oily Dandruff | Dry Dandruff | |
| 15 - 20 | 2 | - |
| 20 - 30 | 7 | 5 |
| 30 - 50 | 11 | 3 |
| 50 - 60 | 2 | - |
3.7. Statistical Analysis
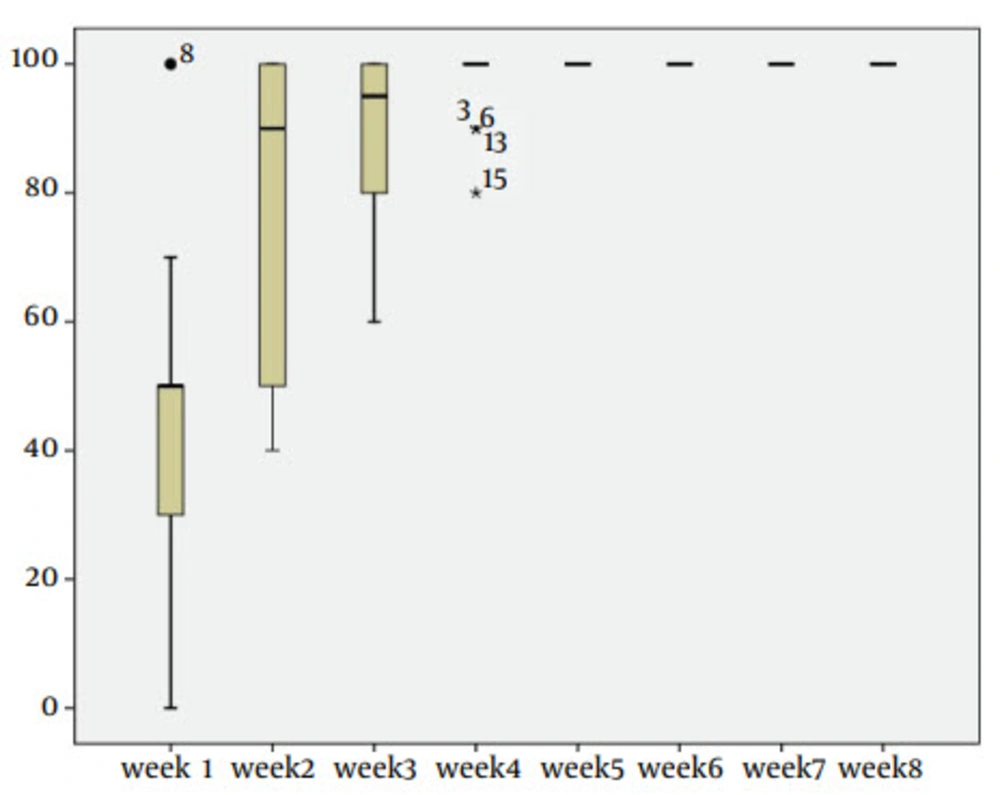
In this study, the SPSS software version 17.0 was used to achieve the results and box plots and mean of dandruff removal plotted. Effectiveness of Shampoo was evaluated during 8 weeks and all 30 patients were carefully checked. The results were statistically significant and a dandruff parameter during 5 weeks was completely removed (box plots shows the issue). In addition, a table of descriptive statistics for better analysis of parameters was plotted, which is showing mean of dandruff decrease and treatment per week (for a series of studies).
4. Results
A total of 13 males and 17 females, enrolled in the study, were closely examined. Results showed remarkable dandruff decrease and itching in the first week. Dandruff was highly removed (approximately it turned to half amount) in 15 people during first week, In fact, about 43% of dandruff were reduced in this week and fully dandruff removal was occurred in the same group during the next 7 days. Other 12 sufferers feel free of dandruff and itching in 1 month (98% of dandruff was treated after about 4 weeks); while 3 remained patients expressed their satisfactory after 5 weeks. Result and investigation about dandruff removal is shown in Figure 1.
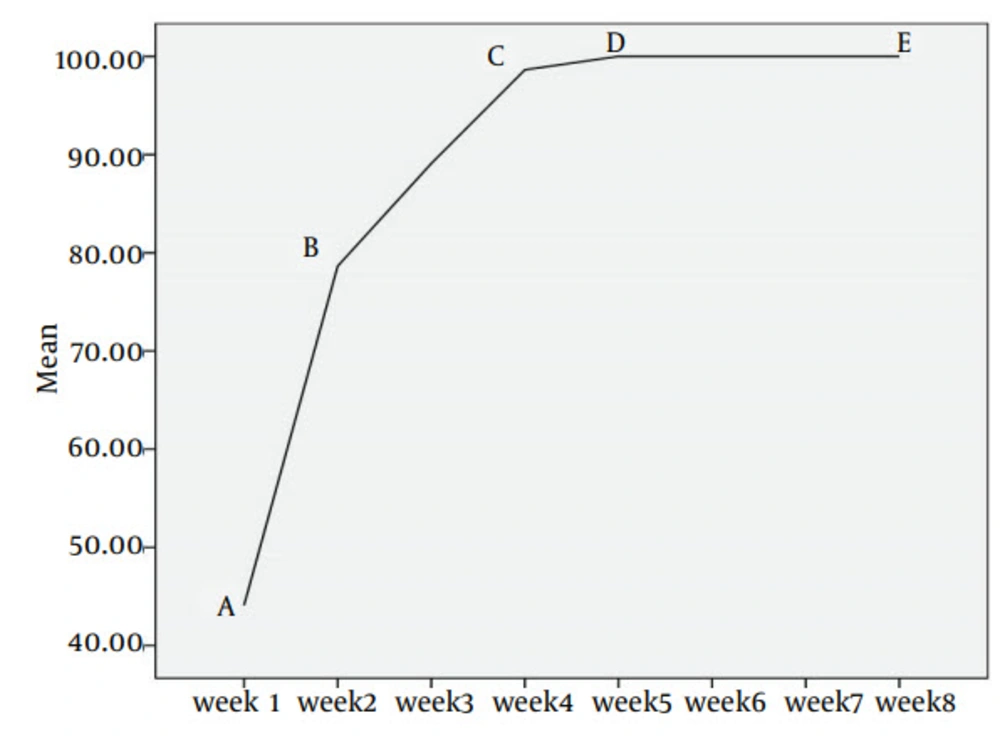
In Figure 2, the dandruff removal accelerating rate is being shown in order to assess the efficacy of treatment due to combining plant extracts and chemical compounds during the two-month experiment.
A-B line shows the most effective treatment in dandruff reduction while using the medicinal combination in first week for all sufferers. B-C line (week 2 - 4) shows fully dandruff removal in more than 95%. The remaining sufferers (5%) show high satisfaction in the fifth week (C-D line). For achieving dandruff removed confirmation after 5 weeks, all 30 patients were observed in weeks 5 - 8 (D-E line) that shows a high ensuring result in dandruff treatment.
The line slop in this figure shows reduction of dandruff. Which in the third week, about 90% were treated and in the fifth week, this amounts increase to 100%. All 30 patients were closely assessed even after fully treatment and satisfaction, till 2 months to be highly sure about nonreturning disease condition. Table 6 shows the average in dandruff removal among 30 sufferers during two months.
| Week | Minimum | Maximum | Mean ± SD |
|---|---|---|---|
| 1 | 0.00 | 100.00 | 43.0000 ± 21.03 |
| 2 | 40.00 | 100.00 | 78.33 ± 23.50 |
| 3 | 60.00 | 100.00 | 89.00 ± 12.96 |
| 4 | 80.00 | 100.00 | 98.33 ± 4.61 |
| 5 | 100.00 | 100.00 | 100.00 ± 0.00 |
| 6 | 100.00 | 100.00 | 100.00 ± 0.00 |
| 7 | 100.00 | 100.00 | 100.00 ± 0.00 |
| 8 | 100.00 | 100.00 | 100.00 ± 0.00 |
aDandruff treatment percentage.
bValid N (listwise) = 30.
Based on the bove-mentioned table, all 30 sufferers were remained in the experiment during two months. Assessment of dandruff removal mean rates in each week showed the reduction of hair dandruff.
5. Discussion
Chemical ingredients are highly effective for treating dandruff, though they have many side effects. On the other hand, medicinal plant extracts with great impact as antidandruff and anti-itching has been shown during centuries, with no side effects, but long- term treatment time needs sufferer’s patience. Base on this fact, PZ+ a highly effective shampoo, combination of 2 scientific sides-chemicals and natural ingredients- have been produced to reach the best results. In this product, Zinc-PCA and PO have been combined with 6 plant extracts inluding P. granatum, R. officinalis, M. chamomilla, U. dioica, M. piperita and S. officinalis.
The zinc-PCA is a combination of zinc and PCA (obtained by cyclization of the L-glutamic acid, amino acid by vegetal origin). This compound reduces unwanted sebum and antidandruff also inhibits activator protein-1 production (involved in producing collagenase and collagen degradation). In addition, PO is an effective antidandruff active which its solubility in cosmetic products depends on pH = 5 ‐ 8. This component is antidandruff, antibacterial and antifungal due to its effect on the yeast cell division. Also, PO is an antifungal ingredient as it inhibits the sodium-potassium channel and reduces dandruff.
Chemical compounds have great influence on removing dandruff; however, inflammation as a side effect is hurting patients. For removing this concern and enhancing the effectiveness, using plant extracts is revealed in pharmaceutical industry. These plants extracts, not only remove dandruff, but also prevent itching and inflammation with no side effects but needs long-term treating persistence.
Punica granatum (includes ellagic acid and β-sitosterol) (15, 16), Rosmarinus officinalis (1, 8-cineole and α and β-pinene) (27, 28) and Salvia officinalis (by having apigenin, ursolic acid, gallic acid and luteolin) possess antidandruff and antifungal properties (39, 40) In addition, caffeic acid and phenolic acid in 2 latter plants decrease chemical side effects and improve treatment while combined with chemicals.
The outstanding point in this combination is due to an anti-inflammatory factor and reduction in chemical adverse side effects. Menthol in M. piperita makes scalp cool and inhibits redness and inflation (36). Also, Matricaria chamomilla by having α-bisabolol as anti-inflammation, blocks cyclooxygenase enzymes involved in synthesizing prostaglandins and inhibits leukotriene formation to prevent redness and it is used as anti-inflammation (32, 33). Interestingly, Punica granatum with cumaric acid is highly effective to reduce inflammation, which is caused by dandruff and chemical ingredients (23, 24), also, several studies are shown that plants contain β-sitosterol such as Urtica dioica by blocking enzyme 5-α-reductase and no chance to for testosterone to turn into Di-hydrotestosterone (DHT),and this will control hair loss (10). One more addition is due to combining 2 chemical ingredients-PO and Zinc PCA that can optimize fast dandruff removal. To sum up, herbal extracts are compounds which enhance antidandruff, anti-itching and anti-inflammatory impacts and inhibit probable adverse side effects of chemicals to increase positive medicinal treatments. Hopefully, it will be great if researchers find out other methods and other medicinal herbal extracts for removing dandruff accompanied with chemical substances. Therefore, it should be said in PZ+, herbal extracts such as Punica granatum has effective antidandruff properties due to punicalagin, also β-sitosterol and ellagic acid are anti-itching and anti-inflammatory compounds. On the other hand, cineole and α-pinene in Rosmarinus officinalis reduce fatty acid peroxidation, results to fungi growth inhibition in pH = 5 - 6. Interestingly, α-bisabolol and luteolin, as anti-inflammations, inhibit cox-1, cox-2 and prostaglandin synthesis, in addition, azulene and coumarin inhibit histamine release. Ursolic acid in Urtica dioica and mentol in Mentha piperita respectively, inhibits 5-α-reductas and promotes blood flow to nourish follicles. To conclude, combination of Zinc-PCA and PO augment resistant dandruff inhibition and herbal extracts reduces chemical side effects; furthermore, it can increase therapeutic effectiveness.
Dandruff is the mild seborrhea usually shows greyish white flakes of skin on the hair and shoulders (these flakes are “oily” or “dry”). Also, this disease may be caused by several different factors such as increased sebum, androgenic, hormonal imbalances, stress and seasonal allergic. The most common symptom of dandruff includes scalp itching and inflammation.
As current therapies cannot completely remove dandruff, herbal extracts with better effectiveness and fewer side effects have been used in cosmetic and pharmaceutical industry. The current study was conducted in the research company of Pars Azmaye Teb (Cerita) which leads to produce and manufacture a highly effective antidandruff shampoo PZ+ to control and remove these white flakes. In modern and effective dandruff treatment, combination of useful chemical ingredients (PO and Zinc-PCA) and extracts of the medicinal herbs is an outstanding soluble to treat this concern, run by the afore-mentioned research company.