1. Background
Gastric cancer is the fourth prevalent cancer and the second reason for cancer-associated mortalities worldwide (1, 2). Chemotherapy is frequently used as a primary treatment for gastric cancer. The main obstacle facing success of chemotherapy is multidrug resistance (MDR), which is characterized by resistance to chemotherapeutic agents after exposure to a chemotherapy drug (3). One of the most important factors contributing to MDR to chemotherapeutic agents is disruption in apoptosis process (4).
Cancers accompanied with changes in proteins that participate in cell death signaling are often resistant to chemotherapy and difficult to be treated by chemotherapeutic agents which function fundamentally through apoptosis induction (5). In gastric cancer, as with other cancers, apoptosis process is disrupted so that it is associated with severe disturbance in BAX/BCL2 system accompanied with a pronounced increase in BCL2 expression (6), loss of APAF-1 expression (7) and inactivating mutation or loss of heterozygosity (LOH) of the caspase 10 gene (8). In addition, p53, p21, Bax and Bcl-2 factors involved in regulation of apoptosis cascades are disturbed in some types of gastric cancer (6, 9, 10). In addition, expression and activity of nuclear factor- κB (NF-κB) transcription factor is pronouncedly increased in human gastric cancer (11). Infection with Helicobacter pylori CagA+ enhanced NF-κB activity (12). Any disturbance in NF-κB activity has been demonstrated to increase proliferation, evasion of apoptosis, genomic instability, increased glycolysis rate and drug resistance in gastric cancer cells, as well as to facilitate tumor invasion and expansion (13-16). Therefore, because of disruption in apoptotic pathways and MDR to chemotherapeutic drugs in gastric cancer, promoting cancer cells toward apoptosis is a useful and practical method of preventing cancer and treating gastric cancer. Moreover, development of drugs aimed to inverse programmed cell death could work against cancer.
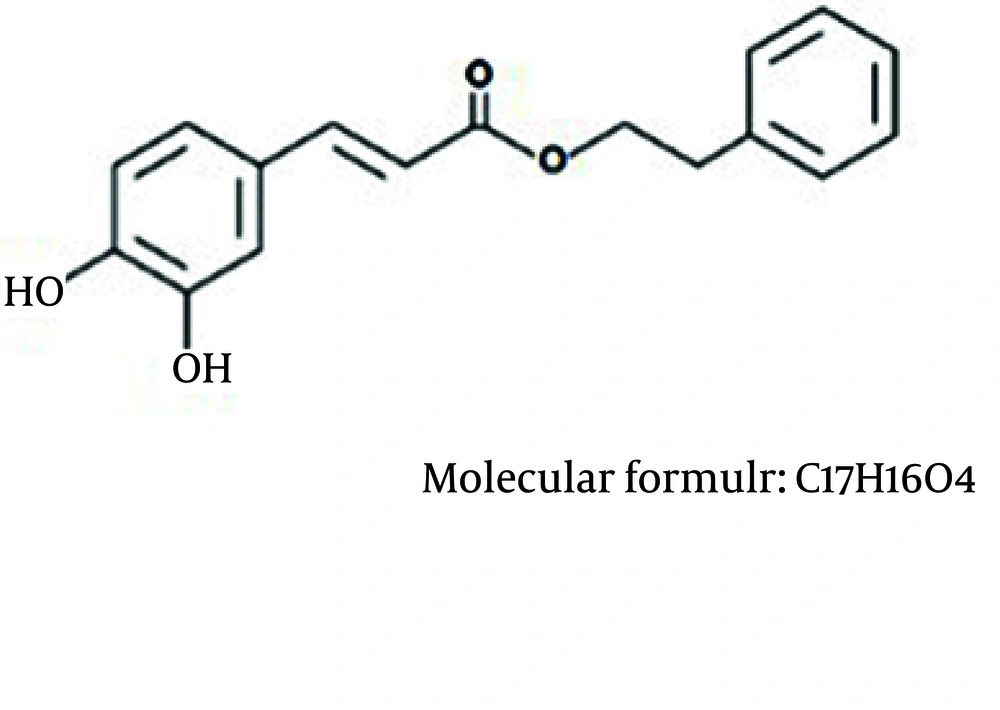
More than 60% of anticancer drugs have been directly or indirectly obtained from natural sources (17). Recent studies have shown promising findings on natural products for treatment and prevention of cancer (18, 19). Propolis is a natural product and a promising source for discovering new drugs (20). Caffeic acid phenethyl ester (CAPE) is an active component of propolis, a resinous mixture obtained from honeybee hives. CAPE was synthesized in 1988 at Columbia University (21) and later found to be a potent anti-cancer ingredient in propolis (22-24). It has important biological activities including antibacterial, antiviral, anti-inflammatory, and NF-κB-inhibitory (25-32).
2. Objectives
The aim of this study was to investigate cytotoxic effect of CAPE and its potential on apoptosis induction in AGS human gastric cancer cells.
3. Materials and Methods
3.1. Chemical and Reagent
Culture medium of Dulbecco’s Modified Eagle’s medium (DMEM), fetal bovine serum (FBS), penicillin-streptomycin and trypsin-EDTA were purchased from Gibco Co (Invitrogen, Carlsbad, CA, USA). CAPE and dimethyl sulfoxide (DMSO) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) Assay Kit was purchased from BIO IDEA Co (Tehran-Iran). FITC Annexin V Apoptosis Detection Kit I obtained from BD Biosciences (Pharmingen, San Diego, CA, USA). CAPE at 100 mmol/mL concentration was dissolved in DMSO as solvent and stored in 100 µL volume at -20°C temperature.
3.2. Cell Culture
In this experimental study, AGS human gastric cancer cell line was purchased from the National Cell Bank of Iran (NCBI), Pasteur Institute of Iran (NCBI, C131). Cells were cultured in DMEM medium containing 10% FBS and 1% penicillin/streptomycin at 37°C temperature in a wet incubator containing 5% CO2. To maintain cells in the exponential growth phase, they were passaged twice a week. All tests were performed in approximately 80% cell confluence.
3.3. Cell Viability Assay
Cell viability was measured by MTT assay kit. The viability of AGS cells was measured in different concentrations of CAPE within 24 hours. Cells were seeded into 96 well plates at a density of 5×103 cells per well and plates were incubated at 37°C temperature in a humidified incubator containing 5% CO2 for 24 hours. After 24 hours, the medium was removed and the cells were treated for 24 hours with medium containing different (0, 10, 15, 20, 30, 40, 50, 60, 80, and 100 µM/mL) concentrations of CAPE. Cells not treated with CAPE were considered as control. Moreover, to neutralize the effect of DMSO, control groups were treated with DMSO 0.1% used as solvent for CAPE. Each CAPE concentration was represented by three wells and replicated thrice. After 24 hours, AGS cells viability was measured by MTT Assay kit according to manufacture protocol. Finally, optical density of each well was read by an ELISA reader (AWARENESS) at 570 nm wavelength and the rate of viability (%) was calculated by the following formula:

3.4. FITC Annexin V/PI Staining
Apoptosis cells were differentiated from live cells or cells undergoing necrosis by FITC Annexin V/PI staining using apoptosis assay kit. To investigate the effect of CAPE treatment on apoptosis induction, AGS cells were firstly cultured in six-well plates with 2 × 105 cells per well in 3.5 mL for 24 hours. After 24 hours, previous medium was removed and the cells with the same amount of culture medium containing 30 µM CAPE were treated for 24 hours. Cells not treated with CAPE and whose medium was replaced with new growth medium were used as control. To neutralize the effect of DMSO, control groups were treated with DMSO, used as CAPE solvent, at 0.1% concentration. The experiments were separately performed for control and treatment groups in three wells in triplicate. At the end of treatment time, adherent and floating cells were collected from both treatment and control groups, rinsed twice using cold PBS and centrifuged. Then, the cells were suspended in binding buffer at 1 × 106 cell per mL. 100 µL of the obtained solution, containing 1 × 105 cells, was transferred into another tube and stained with 5 µL of FITC Annexin V and 20 µL of PI according to apoptosis measurement kit protocol. After 15 minutes incubation of the cells at room temperature and in darkness, 400 µL of the binding buffer was added to the cells and the FITC Annexin V/PI-stained cells were analyzed by flow cytometry instrument (Partec, Germany) using 20,000 cells.
3.5. Statistical Analysis
Statistical analysis of data of MTT measurement for different concentrations of CAPE within 24 hours, calculated as viability (%), was performed by SPSS 19 (IBM Company, United States) using one-way ANOVA followed by Dunnett’s test. The data of apoptosis induction measurement was performed using t-test. P < 0.05 was considered as statistically significant.
4. Results
4.1. The Antiproliferative Effects of CAPE on AGS Cells
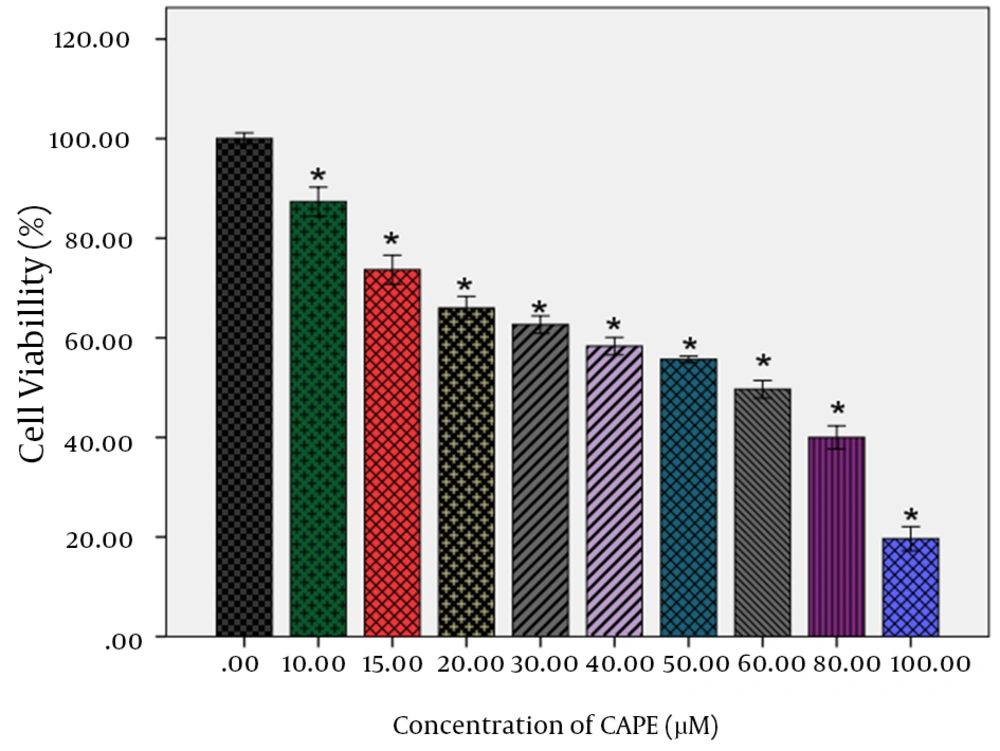
Analysis of data for AGS cells treatment with CAPE within 24 hours indicated that all treated CAPE concentrations decreased the AGS cells viability in a concentration-dependent manner (Table 1). Higher concentrations of CAPE decreased the viability of AGS cells more pronouncedly. For the cells treated with CAPE, IC50 obtained as approximately 60 µM. In addition, a pronounced decrease was observed in the viability of AGS cells treated with CAPE within 24 hours at 80- and 100-µM concentrations, so that the viability was obtained respectively 40% and 19.6% for these two concentrations. Statistical analysis of data on 24-hour treatment with CAPE by Dunnett’s test indicated a statistically significant difference between all treated concentrations and the control (P < 0.001). The effect of CAPE on viability of the AGS cells is illustrated in Figure 2.
The effect of CAPE at 0, 10, 15, 20, 40, 30, 50, 60, 80 and 100 µM within 24 hours was investigated on viability of AGS human gastric cancer cells. Dunnett’s test indicated a significant difference between different concentrations of treatment and control within 24 hours.* P <0.001 versus vehicle.
4.2. Effect of CAPE on Apoptosis
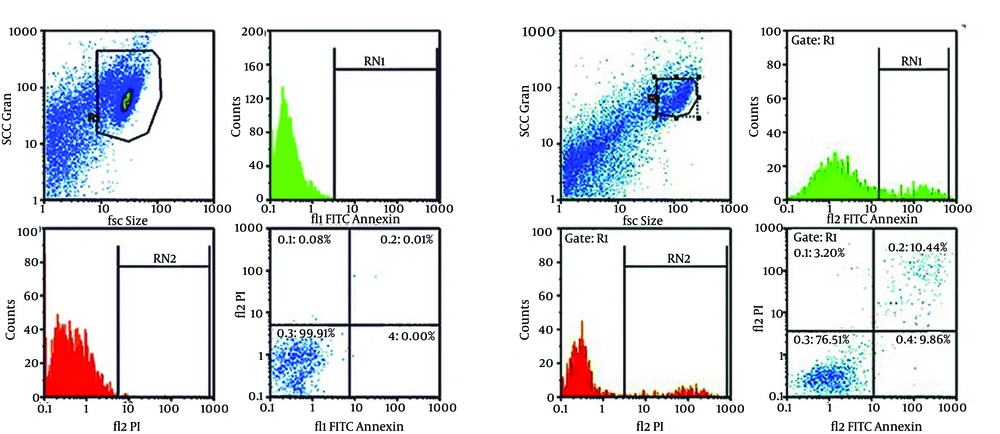
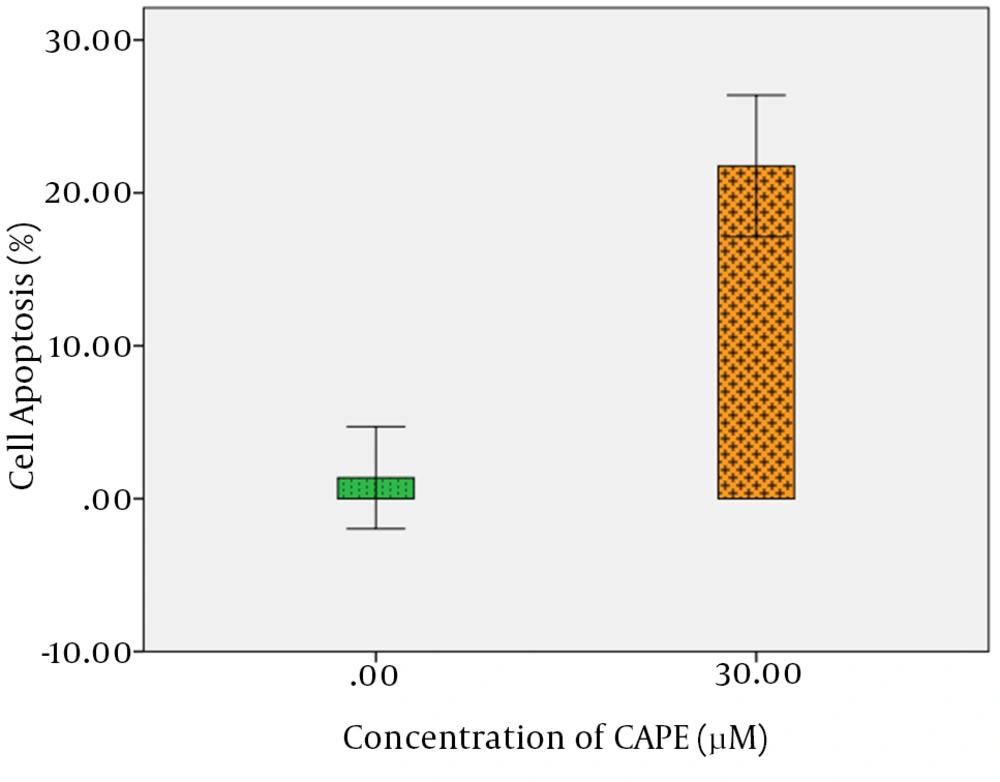
For quantitative assay of CAPE effect on apoptosis induction, detection rate of phosphatidylserine (PS) as a marker of primary apoptosis in the outer membrane was measured by flow cytometry. Compared with the control group, CAPE induced primary apoptosis in AGS cells, so that after a 24-hour treatment of AGS cells with 30 µM CAPE, approximately 9.98% of cells were at primary apoptosis, while in the control group treated with 0.1% DMSO, approximately 0.37% of the cells were at primary apoptosis. In addition, the rate of secondary apoptosis induced by treatment with 30 µM CAPE for 24 hours was 11.78%, while the control group indicated 1% secondary apoptosis. Totally, the rate of apoptosis for treatment with 30 µM CAPE for 24 hours was 21.76%, while it was 1.37% for the control, with a statistically significant difference (P < 0.001) (Figure 3 A and B, Figure 4, and Table 2).
The cells were treated with 30 µM CAPE (B) or growth medium only (control, A) for 24 hours and after binary FITC Annexin V-PI staining, apoptosis was studied by flow cytometry. Necrotic cells lose membrane integrity and allow PI stain to enter the cells. Live cells are FITC Annexin V- and PI-negative. The cells at primary apoptosis stage are Annexin V-positive and PI-negative. The cells at secondary apoptosis stage are Annexin V- and PI- positive.
The mean total apoptosis was measured by assessment of apoptosis using FITC Annexin V/PI staining in treatment and control groups and data was statistically analyzed. t-test demonstrated a significant difference between treated sample and control sample within 24 hours (P < 0.001). Shown data are representative of three independent experiments.
5. Discussion
The findings of this study indicated that CAPE prevented the growth of different cancer cells and CAPE prevented the growth of AGS human gastric cancer cells. The data of AGS cells viability measurement indicated that 24-hour treatment with CAPE decreased AGS viability cells with an IC50 of approximately 60 µM in a dose-dependent manner (P < 0.001). We also studied the role of apoptosis in prevention of AGS cells growth. The data on flow cytometric analysis indicated a pronounced increase in FITC Annexin V staining from 1.37 to 21.76 in cells treated with respectively 0 and 30 µM CAPE, with a statistically significant difference (P < 0.001). Therefore, this study demonstrated CAPE anticancer effect on human gastric cancer cells and increased likelihood of its therapeutic application. Furthermore, these findings suggest that prevention of AGS cells growth induced by CAPE is partially due to apoptosis induction.
Generally, normal cells in response to apoptotic stimulators activate intrinsic apoptotic pathway. Unlike normal cells, cancer cells are exposed to continuous stresses including oncogenic stresses, genomic instability and cell hypoxia and therefore evade this cell response by inactivating apoptotic pathways in most cases. For example, mouse genetic models indicated that genetic inactivation of a BH3-only protein or a caspase caused resistance to apoptosis-specific stimulators and exacerbation of tumor in the mice (33-35).
As mentioned previously, severe disturbances occur in apoptotic pathways in gastric cancer as other cancers. Therefore, regarding the promotion of apoptosis as a therapeutic strategy used to find anticancer drugs, this study investigated the effect of CAPE on apoptosis induction in AGS cells and demonstrated that this compound was able to induce apoptosis in gastric cancer cells. In contrast, some studies indicated that CAPE had no cytotoxic effects on non-tumoral cells including immortal breast cancer cells (MCF-7-10A (ER-)) and peripheral blood mononuclear cells (PBMCs); these findings implicate that CAPE is a viable candidate for cancer therapy without effect on normal cell (3-6).
Although, the mechanism of apoptosis induction by CAPE was not investigated in this study, many investigations assessed different components of apoptosis pathways as potential purposes of CAPE in different cancer cell lines to clarify the mechanism of CAPE activity in apoptotic pathways and develop therapeutic strategies. Orsolić et al. observed a pronounced increase in apoptosis up to 31.24% in MCa cells after 15 hours treatment with 5 or 10 mg/mL CAPE (36). Szliszka et al. recommended that CAPE prevented NF-κB and induced extrinsic pathway of apoptosis in cancer cells induced by stimulation of TRAIL and Fas receptors (37). Kim et al. demonstrated that CAPE amplified TRAIL-induced apoptosis in SK-Hep1 cells through increasing the expression of TRAIL receptors (DR4, DR5) by means of regulation of P38 and JNK signaling (31). However, many studies indicated that CAPE increased caspase 3 and 7 activity in different types of cancer cells (30, 38, 39). In addition, CAPE activates the intrinsic apoptosis through inducing the activity of other proteins involved in proapoptotic processes such as Bax or Bak. However, CAPE decreases the expression of Bcl-2 as an inhibitor of apoptosis (30, 31, 38, 39). CAPE is also involved in activation of intrinsic pathways of apoptosis through releasing cytochrome C from mitochondria into cytosol (31), activating p53 as a proapoptotic protein (30, 31, 40, 41) inhibiting NF-kB (30) and then downregulating IAPs (42). All these effects seem to be independent of the type of tumor cell and concentration and/or dose of CAPE. Therefore, our findings accompanied with those of above-referenced works recommend that CAPE-induced apoptosis in AGS human gastric cancer cell line could be most probably due to inducing extrinsic and intrinsic pathways of apoptosis.
Overall, our findings indicated that CAPE prevents growth and proliferation of AGS human gastric cancer cell line through inducing programmed cell death in AGS cells. Therefore, CAPE could be addressed as a potential chemotherapeutic agent or an adjuvant for treating gastric cancer. Further research could clarify the mechanisms of CAPE effect on apoptosis induction in gastric cancer.