1. Background
Iran is considered rich in biodiversity. It is the home of original stocks of plant species with great commercial and medicinal values traditionally used for healing purposes.
The use of herbal medicine as a complementary and/or an alternative is increasing worldwide (1). However, it has been challenging since identification of active ingredients and analysis of its adverse effects is cumbersome (2). Pomegranate, Punica granatum L., is well-known for many therapeutic and pharmaceutical effects. PPE possess significant antioxidant, antimicrobial, antifungal, antiviral and cytotoxic activity in vitro models. It is also an important source of bioactive compounds. The largest components of the Punica granatum L. fruit extract are tannin and polyphenolics (3).
Middha et al. revealed polyphenolic and nutritive content, antioxidant activity, and phenolic profile of PPE. About five different flavonoids, phenolic acids, and their derivatives including quercetin, rutin, gallic acid, ellagic acid, and punicalagin as a major ellagitannin were identified by high-performance liquid chromatographic (HPLC) method (4).
Punica granatum L. has a long history to treat several conditions including diarrhea, ulcers, aphthae, hemorrhage, and respiratory complications (5, 6). PPE has diverse pharmacological functions such as antioxidant (7, 8), antifertility (9), cytotoxic (10, 11), hepatoprotective (12), and hypoglycemic activities, and tyrosinase inhibition (13, 14).
The study performed by Fawole et al. showed that PPE possesses strong antibacterial, antioxidant and anti-tyrosinase activities. Therefore the PPE could be exploited as a potential source of natural antimicrobial and antioxidant agents (15).
They are also used to treat parasitic and microbial infections. For example, the use of 250 μg/mL pomegranate peel extract (PPE) was most effective to inhibit antibiotic resistant strains of Salmonella typhimurium and Staphylococcus aureus in meat surfaces (16).
Al-Zoreky investigated the use of 80% methanolic pomegranate peel extract, which resulted in reduction of Listeria monocytogenes in fish during storage at 4°C (17). In addition, Shan et al. (18) indicated the effectiveness of pomegranate peel extract to inhibit the growth of L. monocytogenes, S. aureus, and Salmonella enterica in cheese at room temperature. Some other studies reported the use of pomegranate as a taeniacidal drug with high potency (19).
Punica granatum L. is rich in tannins and other biochemical compounds, particularly phenolics; and its peel contains high source of polyphenol compounds, which can reduce inflammation and other disease conditions (20, 21).
2. Objectives
The current study aimed to investigate the in vivo toxicity, safety and tolerability of pomegranate peel extract using BALB/c mice. This study allowed us to have a baseline for more in-depth studies on efficacy and safety of pomegranate peel extract for a possible application in the public health.
3. Materials and Methods
3.1. Plant Extraction
The sour malas cultivar of Persian Punica grantum L. peel used in the current study was collected from mature fruits grown in the Agricultural Research Center of Saveh, Iran. The results obtained in the previous studies demonstrated that among the various tested extract, sour malas peel extract showed maximum antifungal activity against various Candida strains. To prepare PPE, pomegranates were manually peeled, dried and powdered by a mixer grinder. Then, 50 grams of dried peel powder was added to 500 mL of 80% methanol for 10 days in a Soxhlet extraction apparatus. After extraction the solvent was removed, by means of a rotary evaporator, yielding the extracted compound. The stock peel extract was kept in sterile containers at 4°C until use.
3.2. Animal
A total of 25 BALB/c mice were prepared from the animal house of the Pasteur Institute of Iran, Tehran. Adult healthy female animals, eight to ten weeks old, weighing 22 - 25 g were used for animal studies. The mice were randomly grouped; each experimental group consisted of five animals. The animals were kept under standard laboratory conditions of temperature (25 ± 2°C), humidity 50% ± 5% and light/dark cycle (12 hours/12 hours). They were provided with a nutritionally adequate standard laboratory diet. The animals were housed in sanitized polypropylene cages containing sterile paddy husk as bedding. They had free access to standard pellet diet and water.
3.3. Experimental Design
The 22-day dietary toxicity study was performed to evaluate the repeated dose toxicity of PPE in twenty five BALB/c mice according to the standard protocols of organisation for economic Co-operation and development (OECD) guidelines 425 (22). Repeated doses were applied for a total of 22 days to determine the toxicity, tolerance or mortality. They were randomly assigned to five groups of five mice. The methanolic extract was gavaged to BALB/c mice in three doses, and injected intradermally in one dose. Groups of 20 female mice were administered 0.5, 1.9 and 7.5 mg pomegranate peel extract/kg body weight was diluted in a distilled water solution by gavages, six days per week for three weeks. One group received distilled water daily (2.8 mL/kg/daily) by gavages orally and was considered as the control. The doses were administered by oral route in mice as scheduled in OECD guidelines 425. In order to investigate the allergic reaction of P. granatum L. (pomegranate) peel extract, the single intra-dermal injection (224 mg/kg) was done.
Symptoms of toxicity and mortality were observed 24 hours after the administration followed by daily observation for 22 days. In vivo parameters, clinical chemistry parameters, hematology and pathology evaluations were conducted after 22 days.
3.4. Histopathological Sampling and Biochemical Analysis
Blood was collected intracardially from mice under ketamine (100 mg/kg) and xylazine (5 mg/kg) anesthesia and the separated serum was used for the estimation of glucose, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and high-density lipoprotein (HDL) to determine the toxicity of pomegranate peel extract after 22 days of administration.
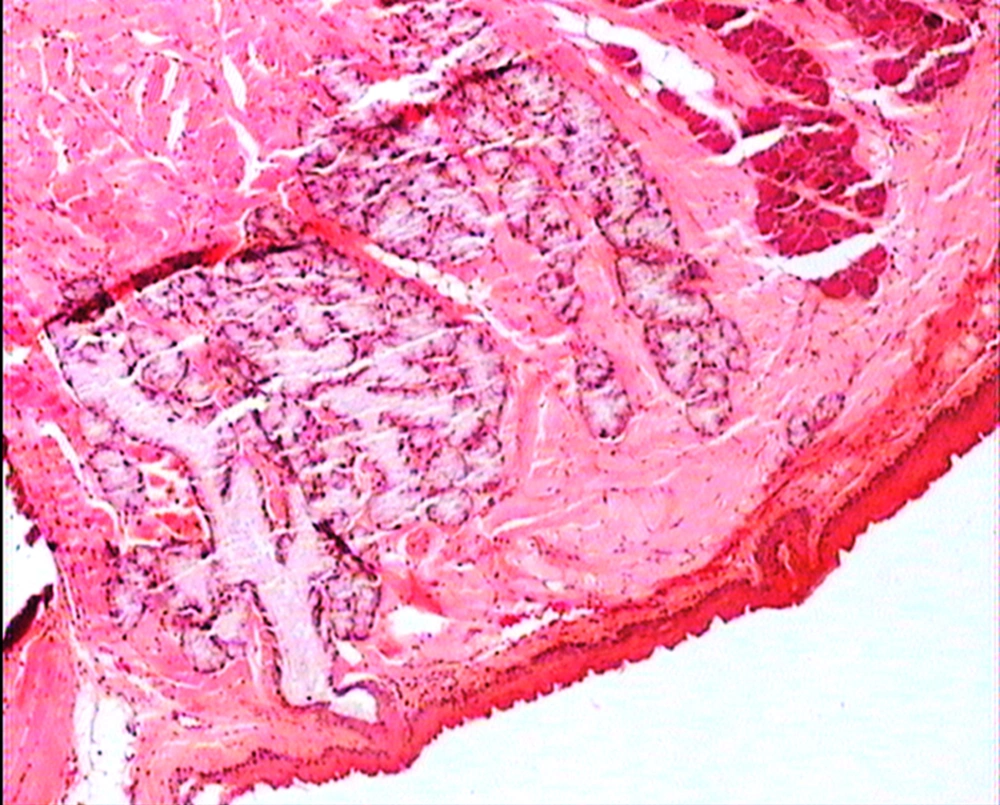
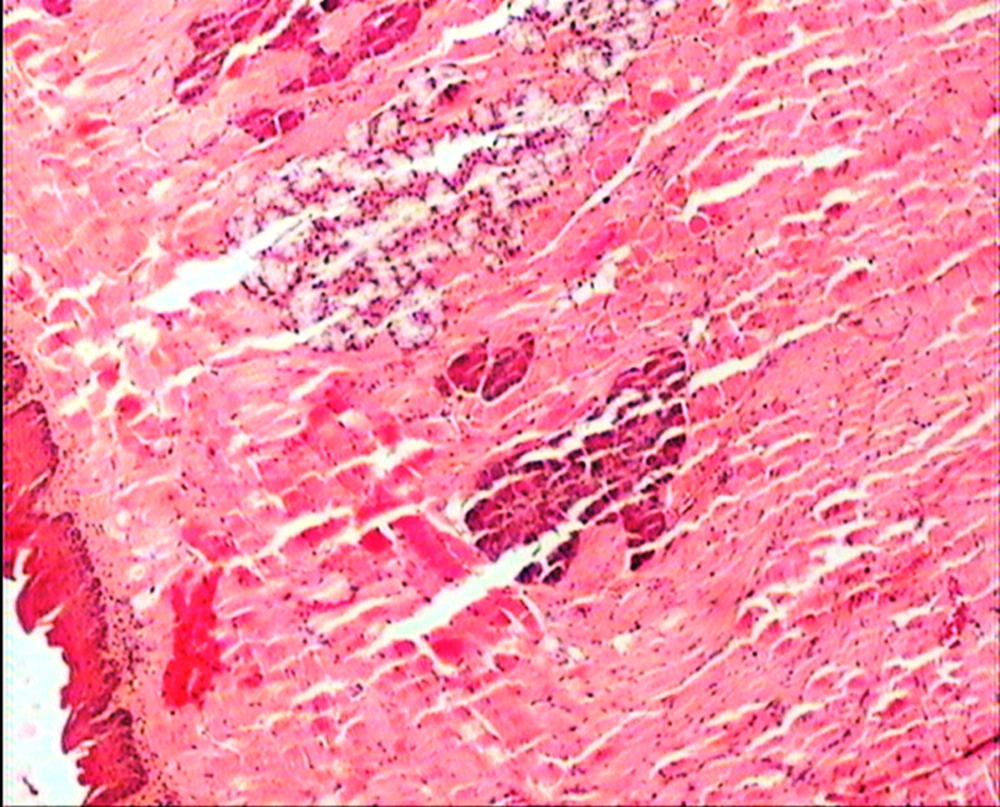
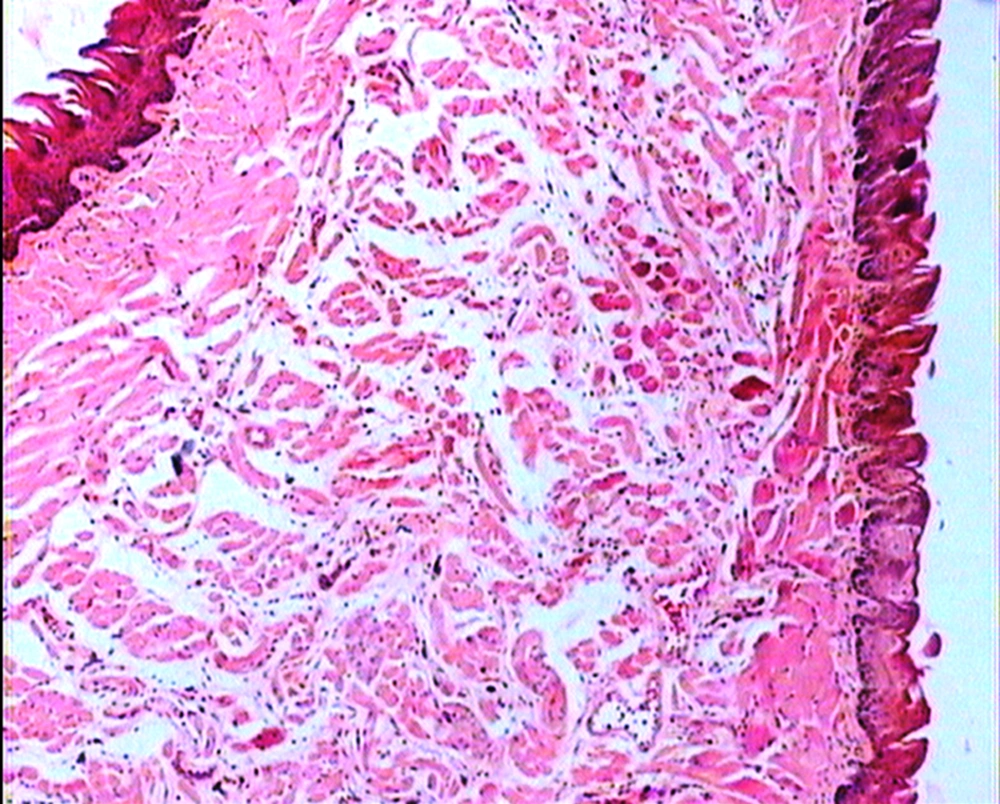
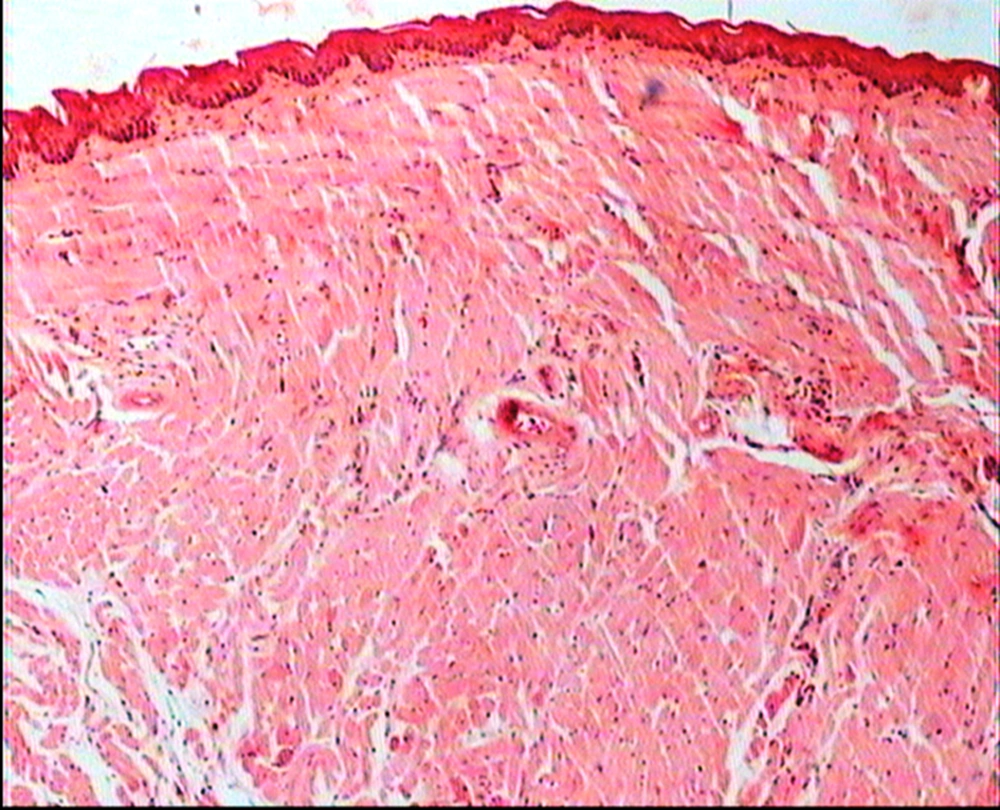
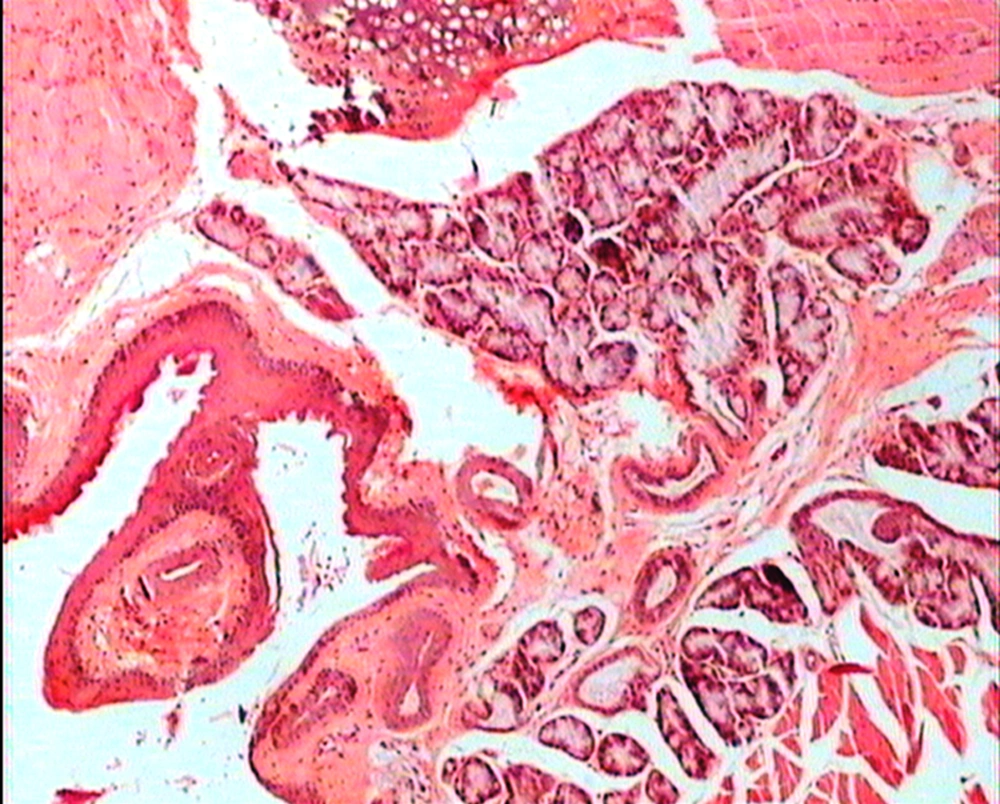
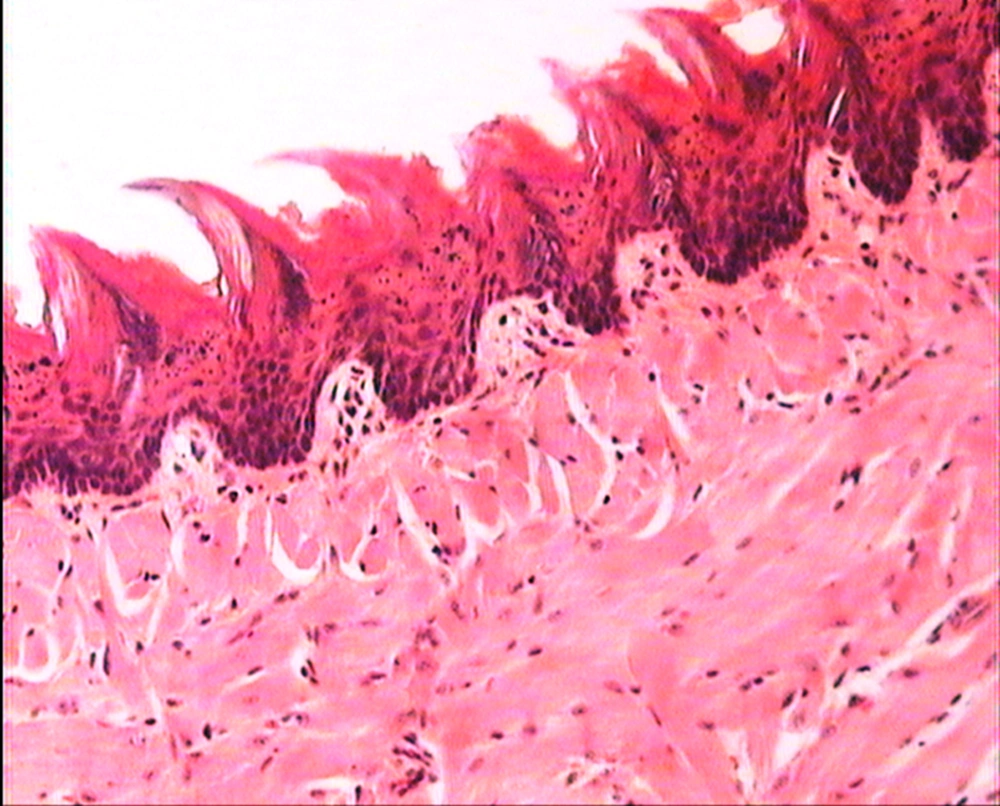
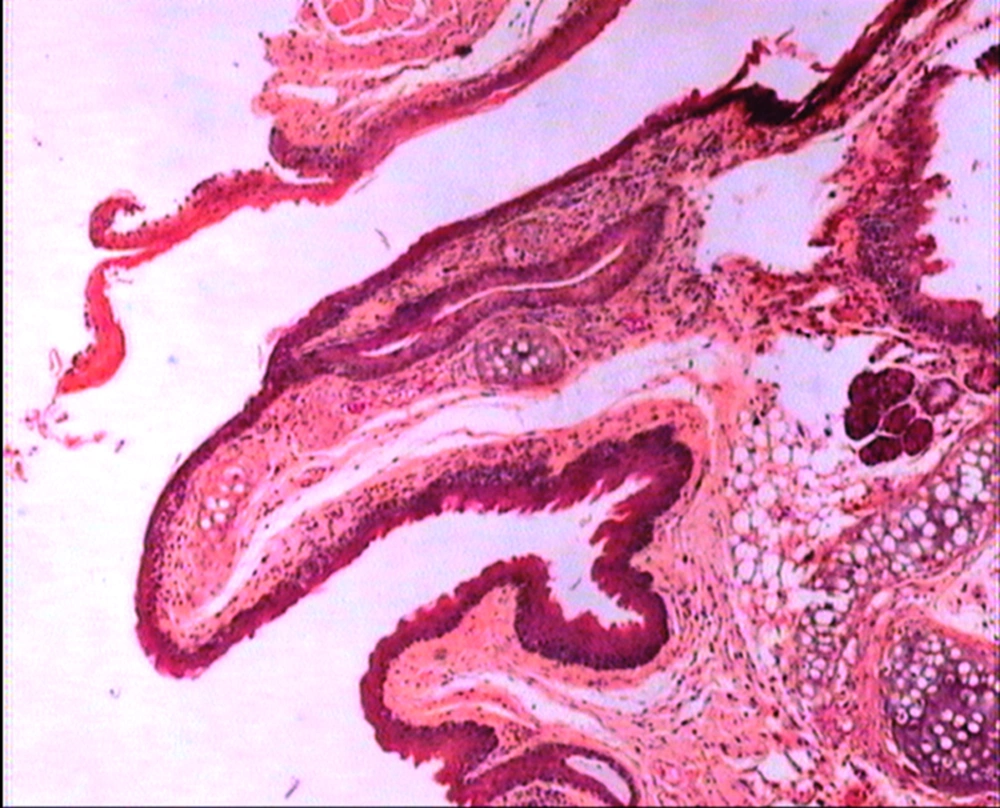
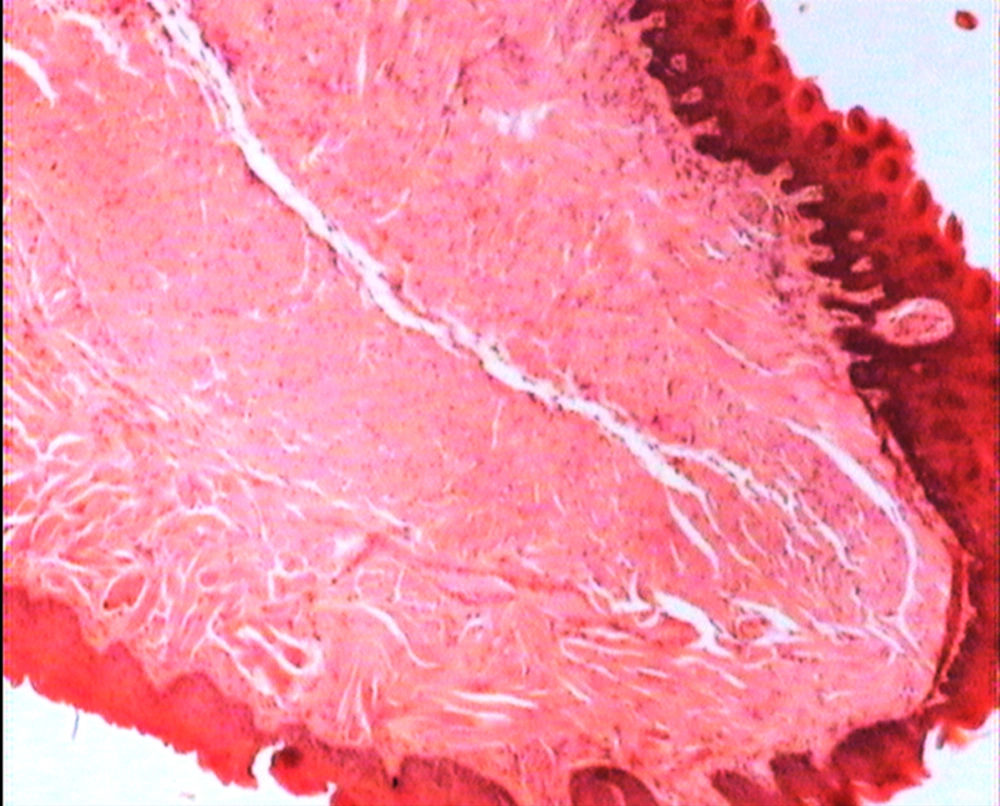
The animals were killed 24 hour after administration of the last gavages. Macroscopic examination of tongue, trachea and larynx tissues was performed after which they were quickly washed in isotonic saline and were collected and fixed in the neutral buffer contained formalin, distilled water, and phosphate mono and desodic (Na H2O, Na2 H2O) for histopathological examination. Histopathological evaluation of the epithelial cell layers of larynx, trachea and tongue were performed to determine the possible effects of cells exhibited by the pomegranate peel extract.
3.5. Statistical Analysis
Data were expressed as Mean ± standard error (SE). The student t-test was used to determine the significance among groups. A value of P < 0.05 was considered to be significant.
3.6. Ethical Considerations
All experimental procedures were reviewed and approved by the Animal Ethical Committee of Pasteur Institute of Iran, Tehran, prior to the initiation of the experiment and the laboratory animals were taken care of according to the committee for the purpose of control and supervision of experiments on animals (CPCSEA) regulations.
4. Results
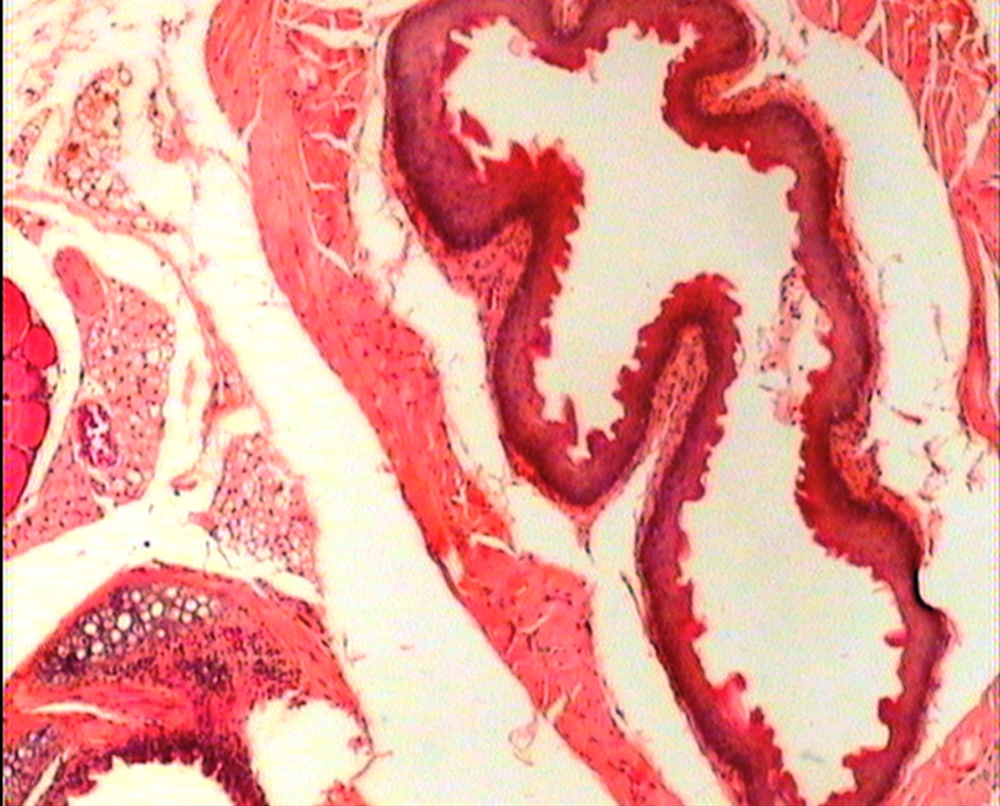
The repeated oral administration by gavage produced no toxic effects in terms of weight gain, food intake, behavioral or biochemical parameters and irritation, inflammation and bleeding in the oral cavity or histopathological of epithelial cell layers of tongue, larynx and trachea. Administration of doses of 0.5, 1.9 and 7.5 mg/kg of pomegranate peel extract in BALB/c mice did not cause death in any of the animals. None of the animals exhibited any behavioral changes, and no toxic signs or symptoms were observed. Biochemical studies revealed no disturbances in glucose, cholesterol, ALT and AST following the administration of pomegranate peel extract. Punica granatum peel extract administered at the supra therapeutic dose (7.5 mg/kg) showed normal histopathological examinations with no inflammation. None of the animals developed any hematologic or biochemical abnormalities. All mice survived to the end of the study. No allergic reaction was shown after 24, 48 and 72 hours.
Figures 1 to 5 show that administration of PPE did not result in any significant changes in histopathological studies of epithelial cells of tongue, trachea and larynx tissues in various doses.
5. Discussion
Although traditional and herbal medicine may promise opportunities in the field of treatment of various diseases, their safety must always be considered. Pharmacological and toxicological evaluations of medicinal plants are essential for drug development (19, 20).
Pomegranate is interesting for researchers because of its chemical composition and biological properties. However, the toxicity of P. granatum is not intensively studied. Fuentes et al. (23) reported that pomegranate fruits were not toxic but pomegranate roots and bark were toxic. Amorin observed no toxic effects in mice treated with aqueous pomegranate extract (24).
According to the 22-day gavage study with repeated doses in BALB/c mice, the use of methanolic extract of P. granatum peel showed no toxicity when directly administered via the oral cavity under 7.5 mg/kg. The results obtained in the previous studies demonstrated that among the various tested extract, sour malas peel extract showed maximum antifungal activity against various Candida strains. Authors selected three doses to evaluate the PPE sour malas cultivar toxicity. The intermediate dose of PPE was the minimum inhibitory concentration (MIC) effect dose against Candida sp. in our previous study (25), we chose three doses to evaluate the pomegranate toxicity. The intermediate dose of pomegranate peel extract was based on the MIC effect doses against Candida sp. (25). Two other doses were then adjusted; the low dose did not induce any toxic responses and the high dose was sufficiently high to induce toxic responses in animal tests.
On the other hand, Vidal et al. (26) reported that the LD50 of 731 mg/kg was determined in mice after intra-peritoneal administration of P. granatum hydro alcoholic extract. The difference could be due to the fact that Vidal et al. used much higher doses than the ones used in the current study. The results of P. granatum peel extract tested on brine shrimp assay by Mehru et al. (27) indicated that the extract had a LC50 value greater than 1 mg/mL which is the recommended cutoff point to detect cytotoxic activity (28). Tripathi and Singh (29) found no statistically significant differences in LC50 values in snails (Lymnaea acuminata) after using various methods of extraction and different parts of the P. granatum plant. Overall, these studies may indicate that the extract of P. granatum were not toxic.
The genotoxicity of P. granantum was reported by Settheetham and Ishida who showed that in vitro use of aqueous pomegranate peel extract induced apoptosis in human cells (30). Amorin (24) did not observe genotoxic effects in mice treated orally with aqueous extract P. granantum at dose of l000 and 2000 mg/kg. Overall, it can be concluded that some parts of P. granatum extract may have toxic effect which could be related to its alkaloid content (29, 31) or pseudo pelletierine content (31). In the current study histopathological evaluation of tongue, trachea and larynx tissues exposed to pomegranate peel extract did not show remarkable morphological alterations such as cell disruption and atrophy when compared to the control group (Figures 1 - 9).
Other investigators observed the presence of ellagitannins, tannins and ellagic acid or their derivatives in P. granatum with anti-proliferative and apoptosis activities in many human cancer cell lines such as T24 (bladder), CaSki (cervical), MOLT-4 (leukaemia), MCF-7 and Hs 578T (breast), and DU 145 (prostate) cells (32-34). The study by Pena (35) on chick embryo showed that Punica granatum extract was not embryotoxic at the dose of 0.1 mg per embryo.
In conclusion, the lack of any adverse effects of pomegranate peel extract following its administration in mice suggested that this herbal product is safe and could be used to treat certain oral infections. The effectiveness of this extract against various fungal and bacterial infections is being determined.