1. Background
Cutaneous wound is indicated by an injury to the skin, following trauma or contusion (1). It has been evidenced that wound healing process consists of four phases: hemostasis, inflammation, proliferation, and remodeling (2). Accordingly, cytokines, such as platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), and interleukin (IL)-1β stimulate re-epithelialization, angiogenesis, granulation tissue formation, fibroblasts proliferation, and collagen synthesis, in the skin wounds (3, 4). The faster recovery and less scarring still represent the most important goals in wound healing. Moreover, shortening of wound repair process is critical, because it reduces the risk of infections and complications, and also is considered cost beneficial (5).
There has been a rapid-growing utilization of herbal medication, which has attracted attention of researchers (6-10). Quince (Cydonia oblonga Miller, Rosaceae family) is known as an important fruit species, with high nutrient value and several advantageous for human health (11, 12). Quince seed mucilage is produced shortly after the exposure of seed coat to water (12). Quince seed mucilage has antioxidants, antibacterial and antifungal effects (13, 14). The investigators have documented that quince seed mucilage could lead to epithelial proliferation, release of VEGF and, subsequently, angiogenesis, deposition of connective tissue, and promotion of wound contraction (15, 16).
Phenytoin is a non-sedative anticonvulsant drug (17). Based on the observation of induced gingival hyperplasia by phenytoin, it might accelerate neovascularization, growth of connective tissue and, consequently, wound healing (18, 19). In addition, phenytoin stimulates fibroblast proliferation, granulation tissue formation, growth of hair follicles, tolerance of tissue elasticity, and inhibits of glucocorticoid production (17, 20, 21).
2. Objectives
According to literature review, there was no human study regarding the role of topical quince seed in wound restoration. Therefore, we decided to conduct a clinical trial to compare the efficacy and safety of topical quince seed with phenytoin, in skin wound healing.
3. Patients and Methods
3.1. Study Design
This double blind, randomized controlled trial was conducted at a dermatology clinic in Ahvaz city, southwest Iran, from September 2012 to November 2013.
3.2. Study Population
Fifty patients with skin ulcer, caused by punch biopsy, were enrolled into the study. They were allocated randomly in two groups (25 patients in each group). The patients were excluded from the study if there was a history of hypersensitivity to phenytoin, immune suppression (cancer, HIV), autoimmune disorders requiring immunosuppressive therapy, malignancy, and pregnancy. The ethical committee of Ahvaz Jundishapur University of Medical Sciences approved the study and all patients signed informed consent forms.
3.3. Intervention
We used a disposable biopsy punch of 5 mm for the patients who needed cutaneous biopsy to confirm their dermatological diseases. Treatment group received 5% quince seed cream. It was prepared in the department of pharmacology and toxicology at Ahvaz Jundishapur University of Medical Sciences, as described by Hemmati et al. (22). On the other hand, the control group received 1% phenytoin cream (Abidi pharmaceutical laboratory, Tehran, Iran). All patients were asked to apply the creams to their ulcers, twice per day. Both creams were identical in the appearance, color and texture, and, therefore, both patients and investigator were blinded to the treatment allocation throughout the study.
3.4. Outcome Measure
Demographic data and characteristics of the patients including age, sex, smoking, underlying diseases, and drug history were recorded using a questionnaire. Healing of ulcers was evaluated before and during the study (on the 3rd, 7th, and 14th days), with respect to the size of the ulcer. We used a calibrated transparent paper to record the exact surface area, then scanned for evaluation using computerized planimetry digital image processing software (Florida, USA) to calculate the exact size of ulcers (23). Our criteria for wound healing included reduction of wound surface area to less than 0.03 cm2, based on the aforementioned software. Also, adverse events of two medications were recorded.
3.5. Statistical Analyses
All data were computerized and then analyzed using SPSS 11.5 (SPSS Inc., Chicago, IL, USA). Analytic statistic tests including T test, Chi-square, Man-Whitney, and repeated measure ANOVA were used. A P < 0.05 was considered significant.
4. Results
There was no significant difference between the two groups in baseline demographic data and characteristic (P > 0.05; Table 1). Two patients from the phenytoin group and three from the quince seed group were dropped out the study, because of loss at follow-up. Finally, 23 patients in the phenytoin group and 22 in the other group completed the trial.
| Variable | Phenytoin Group | Quince Seed Group | P Value |
|---|---|---|---|
| Age, y | 29.8 ± 7.3 | 27.9 ± 6.9 | 0.37 |
| Gender | |||
| Male | 12 (52.2) | 10 (45.5) | 0.65 |
| Female | 11 (47.8) | 12 (54.5) | |
| Medication | |||
| None | 14 (60.9) | 11(50) | 0.95 |
| Corticoids | 2 (8.7) | 3 (13.6) | |
| Aspirin | 2 (8.7) | 2 (9.1) | |
| NSAIDs b | 3 (13) | 4 (18.2) |
Demographic Data and Characteristics of the Treatment Groups a
There was no significant difference in the mean ulcer size between the phenytoin (0.5258 ± 0.06 cm2) and quince seed (0.5334 ± 0.09 cm2) groups, before the treatments (P = 0.79; Table 2).
| Ulcer Size, cm | Phenytoin Group | Quince Seed Group | P Value |
|---|---|---|---|
| Before trial | 0.5258 ± 0.0604 | 0.5334 ± 0.0906 | 0.740 |
| 3rd day | 0.3069 ± 0.0418 | 0.1702 ± 0.1095 | 0.001 |
| 7th day | 0.1617 ± 0.1720 | 0.0436 ± 0.0292 | 0.003 |
| 14th day | 0.0336 ± 0.0266 | 0.0048 ± 0.0052 | 0.001 |
The Mean of Ulcer Size Before and After the Treatments in Phenytoin and Quince Seed Groups
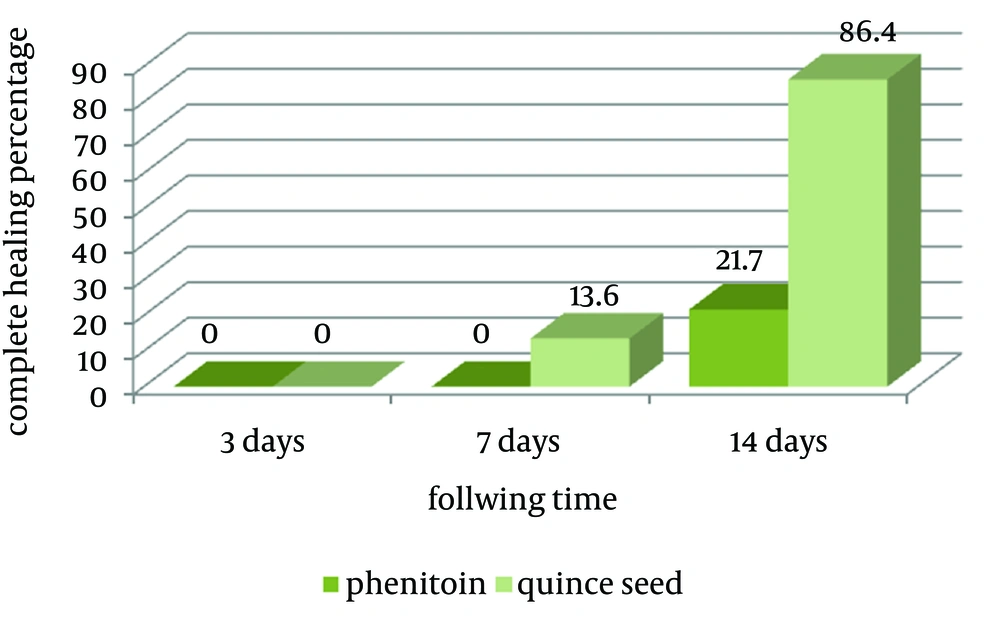
Repeated measure ANOVA analysis did not demonstrated any significant difference between healing processes with age, sex (P > 0.05). There was no complete healing after 3 days in both groups. On the 7th day of treatment, three patients (13.6%) in the quince seed group and no patient in the phenytoin group showed complete healing that was not significantly different according to Fisher exact test analysis (P = 0.11). At the end of the study (14th days), complete healing was observed in 19 (86.4%) and five (21.7%) patients in the quince seed and phenytoin groups, respectively. This result was statistically significant (P < 0.001; Figure 1).
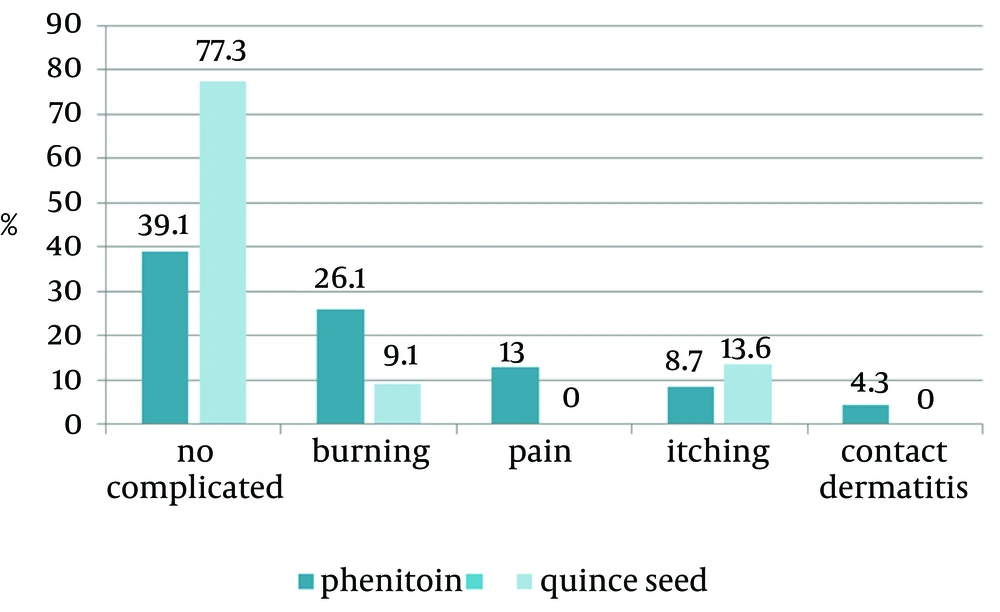
The adverse events consisted of itching, pain, burning and local hypersensitivity and the most common complaint was burning. Complications were observed in 23.7% and 61% of patients in the quince seed and phenytoin groups, respectively, that showed significant difference (P = 0.03; Figure 2).
5. Discussion
Quince seed is an herbal extract with potential healing effects on skin ulcers. To the best our knowledge, there was no clinical study assessing the potential healing effect of topical quince seed on skin ulcer.
When comparing the mean size of the ulcer healing obtained at the 3rd, 7th, and 14th days, there were considerable differences between the two treatments. Accordingly, quince seed group showed superiority in complete healing of skin ulcers. It seems quince seed cream might be more effective than phenytoin cream in the healing of skin ulcers. According to the present study, 60.9% of patients treated with 1% phenytoin cream showed complications, such as burning, itching, and local hypersensitivity, which were higher than in patients treated with quince seed cream (22.7%).
Hemmati and his colleagues studied the efficacy of quince seed 5, 10, and 15% on skin lesions induced by T-2 toxin, in rabbits. They reported that cream containing quince seed 15%, compared with the placebo, had the best therapeutic effect, leading to faster recovery of the lesions (22). Mucilage has no systemic absorption, and therefore is considered to be a safe healing agent, in comparison to other chemical healing medicines, which might have systemic bioavailability and side effects (22). In another in vivo animal study, Tamri et al. evaluated the role of 5%, 10%, and 20% quince seed creams on the full thickness wounds created in rabbits. The researchers showed the superiority of 10 and 20% quince seed creams, compared with eucerine, in wound contraction (24). The possible mechanisms of healing effect of quince seed are proposed, as followed: this herbal medication may play a preservative role by attaching to the surface proteins of wound and facilitating the wound healing (25). On the other hand, quince seed, as a physical barrier, can diminish water evaporation. It may also be a barrier against microorganisms invasion (13). Cydonia oblonga species (including quince seed cream) is an excellent natural source of phenolic acids and flavonoids, which are well known potent antioxidants (25, 26). The phenolic compound in quince has been reported to have free radical-scavenging activities (26, 27). Knowing the fact that antioxidant agents may facilitate wound healing (28, 29), quince seed may exhibit wound healing properties via the aforementioned mechanisms. Other possible theories that can explain the observed wound healing activities of quince seed include: activating the growth factors, induction of the collagen production, and stimulating more blood circulation (16). Therefore, more studies are needed to clarify the underlying mechanisms of the wound healing profile of quince seed.