1. Background
Cydonia vulgaris Pres is a plant, which has been exported to many countries covering almost 70% of the word crop production. Cydonia oblonga Miller also known as Quince seeds is a plant from the Rosacea family, which forms a small shrub and is native to Southwest Europe and some parts of Asia such as Iran (1, 2). It is medically used as a demulcent and for the treatment of asthma and coughing, while it may also be used as a flavoring agent or preservative (3). Mucilage are biopolymers most commonly used as adjuvants in the manufacturing of different pharmaceutical products and include binding, thickening, stabilizing, humidifying (4, 5), disintegrating (6), suspending (7), emulsifying (8), and sustaining properties (9), at different proportions in many pharmaceutical dosage forms. Natural mucilage are preferred over semi-synthetic and synthetic materials due to their non-toxic, low cost, free availability, emollient and non-irritating nature (10). Biopolymers are extensively influenced by many factors such as the chemical composition and molecular structure of the biopolymer. These factors may become very critical with some improper practical conditions and under pressure vacuum processes such as extraction, purification and drying methods while further modifications intensify such problems, thereby influencing the functional properties of biopolymers (11). Quince seeds mucilage is a plant biopolymer with a polysaccharide protein structure containing flavonoids, sterols, alkaloids, tannins, saponins, resin, phenol, and terpenoids (3, 12). Most of these compounds have a heat labile nature specially carbohydrates, which may be sensitive to oven temperatures. The drying process is a critical part of food and drug operations because the plant may undergo undesirable changes in texture, density, porosity and sorption characteristics and the overall quality of the dried product may be altered (11, 13). Usually, dried products are influenced by the type and condition of the drying process, which leads to the removal of a large portion of moisture from the plant (14). For some plant gums the drying techniques that have been used so far are vacuum (15), spray (16), oven, and freeze drying (11, 17, 18). Previous study results showed that using high temperatures for a long time might cause chemical degradation of heat labile compounds or change taste and/or color. Besides, quince seeds have not been dried without the use of ovens thus investigating what may happen to its compounds as well as its physicochemical and mechanical properties after oven drying and comparing this to freeze-drying is important.
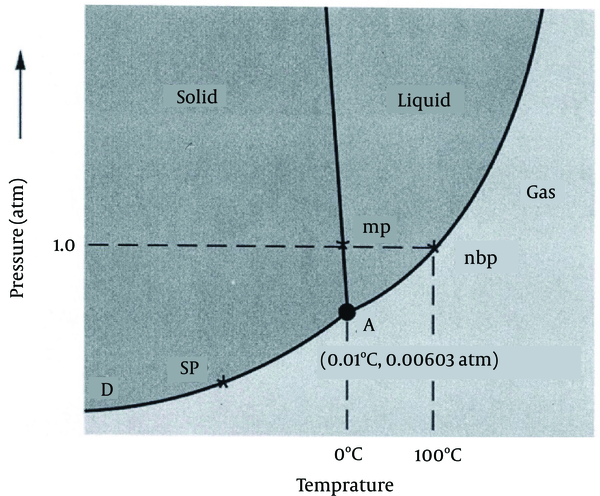
Freeze-drying (lyophilization) is a process of drying in which water is sublimed from the product after freezing. This process is a thermodynamic phenomenon in which phase conversion occurs at a pressure and temperature for each solvent. This physicochemical event will take place under a definite point (A) of equilibrium between three states of liquid (water), solid (ice), and vapor (steam). These situations are shown in Figures 1 and 2 (19).
2. Objectives
The aim of this study was to develop a drying method to promote quince seeds mucilage stability by comparing two systems of cold (lyophilization) and hot (oven) drying regarding physicochemical and mechanical properties of the mucilage powder, for the manufacturing of an antitussive tablet in the future.
Water Phase Diagram With Triple Point A (19)
Water Phase Diagram With Different Ice Stages (19)
3. Materials and Methods
Quince seeds from Isfahan were purchased from a local market and then identified as Pyrus oblong, by the botanical department of the university. All other chemicals and reagents were of analytical grade. Instruments used were a freeze dryer (Vd-60, Japan), hot air oven (AS, Iran), weighing balance (PT 120, Sartorius, Germany), American sieves with different meshes, IR-spectrophotometer (Vertex70, Bruler, Germany), elemental analyzer (Perkin Elmer 11-20400), and Corning pH meter.
3.1. Extraction and Drying of the Mucilage
Quince Seeds mucilage (QSM) was isolated and purified according to a previously described method (10). The seeds (100 g) were dried in sunlight and then an oven (105°C) to retain their properties. Hard and crust materials were treated with petroleum ether to remove pigments and fats, and then dried at room temperature. The remaining materials were macerated in water for five hours and then boiled for 30 minutes and allowed to stand for 30 minutes. Thus the mucilage was extracted in a mild warm water medium. All the material was squeezed from eight muslin bags to remove the marc. Equal amounts of alcohol (95°C) were added to the filtrate for precipitation of mucilage in a rich phase. The yield mucilage was separated and divided to two equal parts. The first part was dried in an oven at a temperature about 50°C. The second part was lyophilized at two consecutive time periods. The first time period was about six hours at -30°C and the second period took about 22 hours at a condition of -50 mTorr pressure and -50°C condenser temperature (20).
3.2. Phytochemical Constituents
The presence of plant chemical compounds such as carbohydrates, protein, flavonoids, sterols, alkaloids, tannins, saponins, resin, phenol, and terpenoids were determined using biochemical identification tests (21, 22).
3.3. Solubility Evaluation
This test was carried out in water and non-polar solutions such as acetone, chloroform, and ethanol in accordance with the British pharmacopeia (BP) specifications (23).
3.4. Swelling Index
In two separate tests 1g of each dried powder (freeze and oven dried) was moistened with 0.5 mL of ethanol (95%) into a 25ml graduated vessel. The final volume was adjusted on 25 ml with distilled water. The vessel was shaken vigorously every 10 minutes for one hour and allowed to stand for three hours. The old and the new volume occupied by the mucilage powder was measured and then the value of swelling index was recorded (23).
3.5. Loss on Drying
A quantity of 1g of the samples (lyophilized and oven dried) was transferred to each of the several petri dishes and then dried in an oven at 105°C until a constant weight was obtained. The moisture content was then determined as the ratio of weight of moisture loss to weight of sample, expressed as a percentage (23).
3.6. Total and Acid Insoluble Ash
The total ash content was estimated by the measurement of the residue left after combustion in the furnace at 450°C. The ash obtained from the test was boiled with 25 mL of 2 M hydrochloric acid solution for five minutes and the insoluble matter was filtered then washed with hot water to ignite and the subsequent weight was determined. The acid insoluble ash was then calculated as a percentage (23).
3.7. pH Evaluation
The pH of 1% w/v dispersion of the samples (lyophilized and oven dried) in water was determined for five minutes using a Corning pH meter (24).
3.8. Microbial Load (Antimicrobial Evaluation)
Microbial amount for both samples of different drying systems was counted for total aerobic microbial colony using the plate count method (25-27). Each freeze and oven dried mucilage sample was poured into a sterile container. Aliquots of 0.01 mL of each sample were diluted in 1.0 mL of deionized water under sterile conditions and then an aliquot of each sample was spread on petri dishes containing blood agar. Plates were incubated inside of a candle jar with 5% - 7% CO2 concentration at 35 - 37°C for 24 hours. The colonies were then counted, separately (27).
3.9. Micromeritics Properties
In this evaluation the physical and mechanical properties of the lyophilized and oven dried mucilage were considered. Angle of reposed, bulk and tapped densities and the compressibility properties on the basis of Carr’s index percentage and Hausner’s ratio were calculated and compared (21, 22, 28, 29).
3.10. FT-IR Spectrum and Elemental Analysis
This study was carried out for both freeze and oven dried mucilage. The purification and recrystallization of the lyophilized quince seeds mucilage powder was compared with the oven dried mucilage powder. The elemental frequency alternation was verified by FT-IR spectrophotometer (Vertex70, Bruler, Germany), using a combination disc of the mucilage powder with dried potassium bromide at a proportion of 1:200. The elemental analysis of carbon, hydrogen and nitrogen in the mucilage was carried out using a Perkin Elmer analyzer.
3.11. Statistical Analysis
The data were presented as mean ± standard error of the mean (SEM). The significance level was calculated by one-way analysis of variance (ANOVA) using the post-hoc test. P values of 0.05 were considered significant.
4. Results
4.1. Yield Value Calculation
The yielded values of 50 gr crude quince seeds mucilage processed by freeze and oven drying were about 3 and 9.25 grams (6 and 18.5%) in powder forms, respectively (Figures 3 and 4). The total time for oven and freezing was about 24 and 28 hours, respectively.
4.2. Phytochemical Constituents
Phytochemical results are shown in Table 1. The screening of chemicals showed positive test results for carbohydrates, the most important ingredient that should be present in mucilage. However, for the other constituents such as those listed on Table 1, and especially for cyanogenic glycoside (amygdaline), the test was negative.
4.3. Physicochemical Properties
The results of the physicochemical properties including solubility, swelling power, loss on drying, total and acid insoluble ash, microbial load, and pH value of both freeze and oven dried mucilage are shown in Table 2.
| Plant Chemical Constituents | Results by Positive or Negative Signs |
|---|---|
| Carbohydrate | + |
| Protein | - |
| Flavonoids | - |
| Tannins | - |
| Saponins | - |
| Sterols | - |
| Alkaloids | - |
| Triterpenes | - |
| Glycosides “cyanogenic” (amygdalin) | < 0.001% |
| Fats and oils | - |
| Resins | - |
| Phenols | - |
| Diterpenes | - |
Phytochemical Screening of Quince Seeds Mucilage
| Parameters | Freeze Dried | Oven Dried |
|---|---|---|
| Solubility | ||
| In water | Soluble | Slightly soluble |
| In non-polar solutions | Insoluble | Insoluble |
| Swelling index in distilled water | 30 ± 3 | 20 ± 2 |
| Loss on drying, % | 4 ± 0.2 | 8 ± 0.3 |
| Total ash, % | 2.5 ± 0.1 | 3.5 ± 0.15 |
| Acid insoluble ash, % | 0.4 ± 0.05 | 1 ± 0.03 |
| Microbial load | ||
| Bacteria, CFU/g | 2 ± 0.02 | 8 ± 0.03 |
| Fungi, CFU/g | 1 ± 0.001 | 9 ± 0.04 |
| pH | 4.9 ± 0.2 | 5.6 ± 0.3 |
Comparative Physicochemical Properties of Quince Seeds Mucilage (Freeze and Oven Dried)
4.4. Particles Movement Phenomenon (Micromeritics)
The density of mucilage powder (bulk and tapped), angle of repose, porosity, compressibility index and Hausner’s ratio, for both lyophilized and oven dried powders are shown in Table 3.
| Parameters | Freeze Dried | Oven Dried |
|---|---|---|
| Bulk density, g/cm3 | 0.5 ± 0.02 | 0.8 ± 0.03 |
| Tapped density, g/cm3 | 0.6 ± 0.03 | 0.82 ± 0.04 |
| Carr’s index, Compressibility % | 10 ± 1 | 2.2 ± 0.02 |
| Hausner’s ratio | 1.01 ± 0.04 | 0.85 ± 0.03 |
| Porosity | 0.07 ± 0.002 | 0.025 ± 0.001 |
| Repose angle, ° | 28 ± 0.18 | 22.0 ± 1.05 |
Micromeritics and Mechanical Properties of Quince Seeds Mucilage (Freeze and Oven Dried)
4.5. FT-IR Analytical Results of Quince Seeds Mucilage
The individual comparison of structural and branch frequency spectrum of quince seeds mucilage dried by freeze and oven drying are shown in Table 4.
| Assignment | Frequency, Cm-1 | |
|---|---|---|
| N-H stretch of primary amine | Oven | Freeze Dried |
| C-H stretch of aliphatic amine | 3408.08 | 3400 |
| N-H bending of primary amine | 2927.32 | 3000.65 |
| C-H bending of primary amine | 1655.86 | 1652.21 |
| C-H bending vibration of - CH2 | 1416.11 | 1414.06 |
| C-N stretch of primary amine | 1380.96 | 1370.65 |
| N-H different stretch of compound primary amine | 1045. 65 | 1050.85 |
| Different stretch of compound primary amine | 950 | - |
| Different stretch of compound aliphatic amine | 820 | - |
| N-H different stretch of compound aliphatic amine | 712 | - |
| C-H different stretch of compound aliphatic amine | 605 | - |
| C-H and CH2 different stretch of compound aliphatic amine | 500 | - |
| Unknown picks | < 500 | - |
FT-IR Chemical Evaluation of Quince Seeds Mucilage Powder (Freeze and Oven Dried)
4.6. Elemental Evaluation
Quince seeds elemental results of freeze and oven dried mucilage are quantitatively compared in Table 5. The element of carbon, hydrogen and nitrogen along with their relations and frequencies (cm-1) are shown in this table.
| Drying Methods | Analysis of Elemental Composition, % | |||
|---|---|---|---|---|
| Nitrogen | Hydrogen | Oxygen | Carbon | |
| Freeze dried | 8.20 ± 0.9 | 9.20 ± 1 | 50.5 ± 3.5 | 32.10 ± 2 |
| Oven dried | 6.3 ± 0.7 | 6.21 ± 0.8 | 48 ± 3.2 | 39.49 ± 2.5 |
Comparative Elemental Analysis of Quince Seeds Mucilage of Freeze and Oven Dried Powder
5. Discussion
The evaluation of phytochemical contents of quince seeds mucilage revealed that there was no difference between the mucilage powders compositions dried by lyophilization or the oven method. Also, mucilage dried by both methods showed the same constituents of essential chemical compounds yet the lyophilized powder had more of a white color compared to the oven dried powder. It should be noted that presence of a white color for a drug is due to purity considering. Compared with the quince seeds dried in the oven (21, 22, 30) the mucilage dried by lyophilization was very soluble in water yet the oven dried powder was slightly soluble, as reported by Patel et al. for the oven dried form (21, 22). Further advantages of this drying system include the fact that freeze-dried products are often more soluble and/or more rapidly soluble. Dispersion products are more stable throughout the shelf life time, when lyophilized the products subject to degradation by oxidation might have a better stability because the process is carried out in a vacuum atmosphere. Also, masking of bitter and/or salty taste of drugs, improvement of purity, and recrystallization of drugs are other applications of freeze-drying.
With respect to swelling power, a high and significant (P < 0.05) swelling level was observed (about 30%) for lyophilized mucilage. Of course, this high level of swelling means that the quince seeds mucilage could be a good and safe plant binder and a disintegrating and matrix agent for sustained release of dosage forms (28-30). Finally, the Iranian quince seeds swelling value is an important quality compared to the non-native forms (21, 22). Consideration of moisture contents is important for understanding the economic importance of an excipient related to drying, packaging and storage, for industrial application (31). Besides, for material for which there is uncertainty about their mineral purity, using the freeze-drying method is preferential as it allows the detection of adulteration. As shown in Table 2, the moisture content of freeze-dried mucilage was as low as 50% less than the oven dried. This significant (P < 0.05) result clearly proves the performance of the freeze-dried method for mucilage containing a large amount of water. Therefore, suggesting a better and more suitable formulation for products containing moisture sensitive drugs. The presence of excessive moisture at a suitable temperature will lead to the activation of enzymes and the proliferation of microorganisms, thereby affecting the shelf life of most formulations (21, 31). Investigation of the moisture content is important because the economic importance of an excipient for industrial application lies not only on the price and availability of its biomaterials but also the optimization of production processes such as the drying method, packaging, and storage conditions (31). The amount of ash is important because it decreases the level of impurity and adulteration in handling of a drug. Results indicating greater than 10% of acid and insoluble ash might be a proclamation for adulteration. It should be noted that adulteration by sand or earth would also be detected as the total ash is normally composed of inorganic mixtures of carbonates, phosphates, and silica. Thus, the low values of both kinds of ash in Iranian quince seeds indicate low levels of contamination. Regarding microbial load, amounts of bacteria and fungi in quince seeds were found to be within official limits yet there was a significant difference (P < 0.05) in the microbial load of the mucilage with respect to the drying method. The pH is an important factor amongst physicochemical properties, which could provide us with good knowledge about the stability and physiological activity of a drug when it is used in a pharmaceutical product (30). Although there was no significant difference (P > 0.05) between the pH of the mucilage obtained by different drying methods yet, it could be concluded that the oven dried mucilage was more neutral in an aqueous medium while the freeze-dried mucilage had better purity and quality.
Comparing the results of the two methods of drying clearly showed a significant difference (P < 0.05) between almost all micromeritic parameters. These factors have an influence on the compaction profile arrangement and flowability of a powder, which is very important in pilot and industrial scale up processes. Evaluation of micromeritic properties of quince seeds mucilage powder showed good flowability and compressibility, especially when the mucilage was freeze dried. Generally, bulk and tapped densities give an insight on the packaging and arrangement of particles of the compaction profile of a mucilage powder (21, 22). That was the reason we investigated mucilage micromeritic and mechanical properties for different processing methods before producing the quince seeds tablet. The Carr’s index and angle of repose of freeze-dried quince seeds mucilage were 10% and 28°, respectively. Compared to the oven-dried mucilage, the freeze-dried form had better flow with popper compressibility (Carr’s index value). In elemental analysis interpretation, the ratio of carbon to hydrogen was about 6:1, indicating a long chain with a good amount of unsaturation due to aromatic rings and/or polysaccharide composition. The low level of nitrogen was suggestive of amino acid compounds (peptide) cross-linked in the samples. Finally, the yield value of freeze-dried mucilage powder was about three folds less than the oven method, yet as it is clearly seen by their pictures (Figures 1 and 2) the oven-dried powder had become brown. This indicates oxidation of some of the materials and therefore, less stability. Natural gums and mucilage are promising biodegradable polymeric materials. They might deteriorate when stored in warm weather or dried in hot conditions. Therefore, they need to be preserved, conditionally (10). Comparing the color of both lyophilized and oven-dried mucilage in Figures 1 and 2, it is obvious that the color of mucilage could be changed by the oven-dried method (Figure 2). Introducing resolutions such as formaldehyde or benzoic acid for protection of stability, which has been done by previous researchers (10), is not desirable and may create toxic material or chemical incompatibility. Therefore, using a cold and safe method, such as freeze-drying is useful, especially in pharmaceutical industries. However, sometimes there is a need to develop or modify existing native natural sources as well as their physicochemical and mechanical properties for a novel drug system.