1. Background
The polyol pathway is a two-step metabolic pathway in which glucose by aldose reductase (ALR2 or AKR1B1; EC: 1.1.1.21) in presence of nicotinamide adenine dinucleotide phosphate (NADPH) is reduced to sorbitol, which is then converted to fructose by sorbitol dehydrogenase and nicotinamide riboside (NAD+) as cofactor (1). Polyol pathway is one of the best candidates that can be responsible for diabetes chronic complications due to cellular toxicity of hyperglycemia since: (I), it becomes active when intracellular concentration of glucose is increasing (2-4); (II), both enzymes of this pathway have presence in human tissues that diabetes complications appear (5);. (III), products of this pathway and changes in cofactors balance can be proponent of the cellular stress that occurs in diabetes (6, 7) and some studies have shown the relationship between increased activity of ALR2 and diabetes chronic complications (8-10). ALR2 is the first and rate-limiting enzyme in polyol pathway, therefore, it is considered as an important potential therapeutic against diabetes chronic complications (11-13). A number of ALR2 inhibitors (ARI) have shown the potency of attenuation or prevention of diabetes chronic complications in animal models (14, 15), and some of them have been investigated in clinical trials (16, 17), but yet most of them have limited success and some of them have severe side effects and low target penetrating (13, 18-20). The most current classes of synthetic ARI evaluated in phase-III trials are carboxylic acid inhibitors (zopolrestat, ponalrestat and tolerestat) and spiroimide inhibitors (sorbinil); the former have shown low target permeability and are not efficacious in vivo and the latter although have better target penetrating ability but, have shown skin reaction and liver toxicity (15, 21, 22). Aldehyde reductase (ALR1; EC: 1.1.1.2) is another member of aldo-ketoreductase (AKR) family and is very similar to ALR2 in sequence, structure and reaction. ALR1 plays a role in reactive aldehyde detoxification (23-25). Unspecific inhibition is one of the restrictions of ARIs and a good inhibitor should inhibit ALR2 more than ALR1 (24, 25). Due to this reason, studies should continue to find an effective ARI. Propolis is a Greek word that means defense from city and is a resinous material that honey bees make and use in hives for sealing hive imports and protection of hives against pathogen microorganisms (26, 27). Propolis chemical compositions are very complex and closely related to plant species that honeybees are nourishing. So far, more than 300 of its compounds are identified. The main chemical classes found in propolis are flavonoids, phenolic, tannin and various aromatic compounds. In addition, propolis contains many of the B-complex vitamins, important minerals and trace elements (28). Propolis is used by humans in folk medicine from ancient times and numerous studies have shown its anti-cancer (29-31), free radical scavenging activity (32), anti-inflammatory (31), antimicrobial (14, 15), and antioxidant (32) properties.
2. Objectives
The current study aimed to evaluate the inhibition of partial purified bovine lens ALR2 by hydroalcoholic extract of propolis (HAEP); characterize the selectivity of HAEP for ALR2 inhibition compared with ALR1,and also investigate the effect of HAEP on intracellular sorbitol accumulation in human red blood cells (RBCs) under high-glucose condition (ex vivo).
3. Materials and Methods
3.1. Chemicals and Reagents
SephadexTM G25, DEAE-cellulose, NADPH, NAD+, sorbitol dehydrogenase (SDH) (sheep liver), D-sorbitol, DL-glyceraldehyde, D-glucose, Krebs ringer bicarbonate buffer, 2-mercaptoethanol (2ME), dimethyl sulphoxide (DMSO), ethylenediaminetetraacetic acid (EDTA), Tris-HCl and ammonium sulfate were purchased from sigma chemical company. All other materials were obtained from Merck Company.
3.2. Isolation of Aldose Reductase from Bovine Lens
Bovine eyes were collected from Ahvaz slaughterhouse. Lenses were isolated rapidly on the ice and then stored at -70°C for next processes. Lenses were mechanically homogenized at 4°C in five volumes of 50 mM potassium phosphate buffer, pH 7.2, and containing 2ME (5 mM). Then the homogenate was centrifuged) centrifuge, Damon-USA (at 10000 g for 30 minutes at 4°C. Supernatant was separated and then adjusted to 60% saturation by adding ammonium sulfate slowly whereas stirring (stirrer: RH basic 2IKA®) on ice, this mixture was kept at 4°C overnight, then centrifuged at 10000 g for 30minutes; then the supernatant was adjusted to 70% and 85% saturation by ammonium sulfate similar to the previous stage. After centrifuging of the 85% solution, supernatant was discarded and the pellet was dissolved in a minimum volume of the above buffer. Then, solution was dialyzed against 5 liters of the same buffer overnight at 4°C. After dialysis, the enzyme was concentrated against solid sucrose. Ammonium sulfate and sucrose were then removed by chromatography on sephadexTM G25.
3.3. Purification of Aldehyde Reductase from Bovine Kidney
Bovine cortex kidney ALR1 was partially purified. Briefly, cortex was separated from fresh bovine kidney and then homogenized mechanically in three volumes of 10m M sodium phosphate buffer, pH 7.2, containing 0.25 M sucrose, 2 mM EDTA and 2.5 mM 2ME. The homogenate was centrifuged at 10000 g for 30 minutes at 4°C. Then, supernatant was subjected to ammonium sulfate precipitation. The precipitate was obtained at 45% and then 75% in a procedure similar to that of ALR2. The pellet of 75% solution was dissolved in minimum volume of sodium phosphate buffer and dialyzed against 5 liters of the same buffer overnight at 4°C. DEAE-52 resin (31 mg/mL) was added to the dialysis product on stirrer at 4°C gently and then, removed by centrifugation. The supernatant was used as the source of ALR1.
3.4. HAEP Preparation from Raw Propolis
Propolis sample was obtained from honeybee hives, located in Kermanshah, Iran. 100 g of frozen propolis was grained and dissolved in 70% ethanol (25 g/mL). This mixture was kept in the incubator at 37°C for two weeks in a dark bottle with continuous stirring. After this period, suspension was filtered twice with Whatman number 4. The final filtered solution was dried by freeze-dryer to obtain crude HAEP. HAEP was dissolved in water, ethanol (EthOH), ethyl acetate (EthAc), CH2Cl2, n-hexane and chloroform at 1 mg/mL final concentration.
3.5. Enzyme Assay
The activity of ALR2 and ALR1 were assayed spectrophotometrically (UV visible spectrophotometer, RH basic 2IKA®, Unico-USA) by measuring the decrease of NADPH absorption at 340 nm as a function of time at 37°C, using glyceraldehyde as substrate. Total protein in the enzyme preparations was determined according to lowery method. The assay mixture in 1ml contained equal units of enzyme, 50 mM sodium phosphate buffer (pH 6.2), 10 mM DL-glyceraldehyde, and 0.3 mM NADPH, with or without inhibitor. The reaction was initiated by adding glyceraldehyde and incubated for 5 minutes at 37°C and stopped by adding 0.5 N hydrochloric acid and then the change in the absorbance was calculated. For inhibition studies, HAEP in the mentioned solvent was tested and the best solvent was selected for subsequent stages.
3.6. In Vitro Incubation of RBC
Venous blood (5 mL) was collected in glass tubes containing 10.5 mg of EDTA. RBCs were separated by centrifugation and washed three times with isotonic saline at 4°C. Washed RBCs were suspended in Krebs-ringer bicarbonate buffer, pH 7.4. Duplicate samples were incubated at 37°C in the presence of 5% CO2 for three hours under normal (5.5 mM) and high (55 mM) glucose conditions. The effect of HAEP on sorbitol accumulation was evaluated by incubating RBCs with 1mg/ml of the selected fraction of HAEP in the enzyme inhibition process.
3.7. Estimation of Sorbitol in RBC
Distilled water, 5 mL, was added to 1 mL of RBCs (washed RBC after incubation) and the mixture was shaken vigorously for 10 seconds to lyse the cells. After that, 1 mL of ZnSO4 solution (0.3 M) and 1 mL of NaOH solution (0.475 M) were added to the cell-lysate to precipitate proteins. The supernatant was separated by 10 minutes centrifugation at 2000 g. The sorbitol content of the supernatant was measured by a fluorometric method.
3.8. Statistical Analysis
All the statistical analyses were performed by SPSS software version 15.0 (SPSS, Inc., Chicago IL, USA). The results were presented as mean ± SD of three independent replicates. Mean differences of the three independent replicates were determined by non-parametric method. P-values less than 0.05 were considered statistically significant.
4. Results
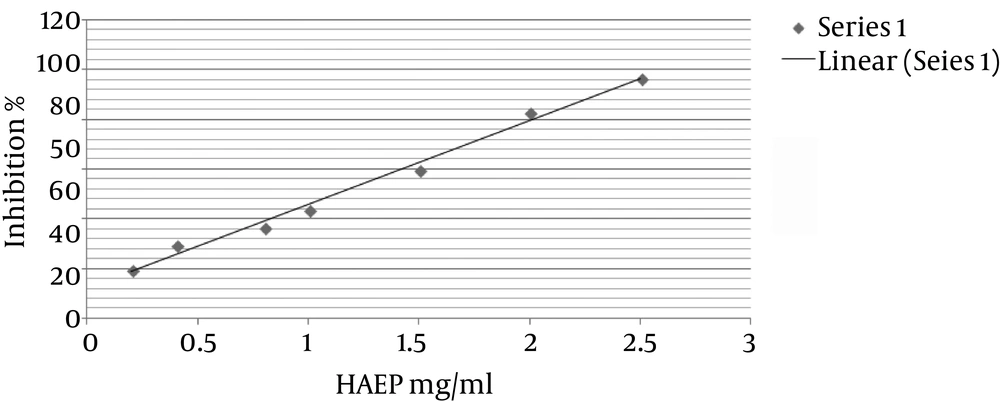
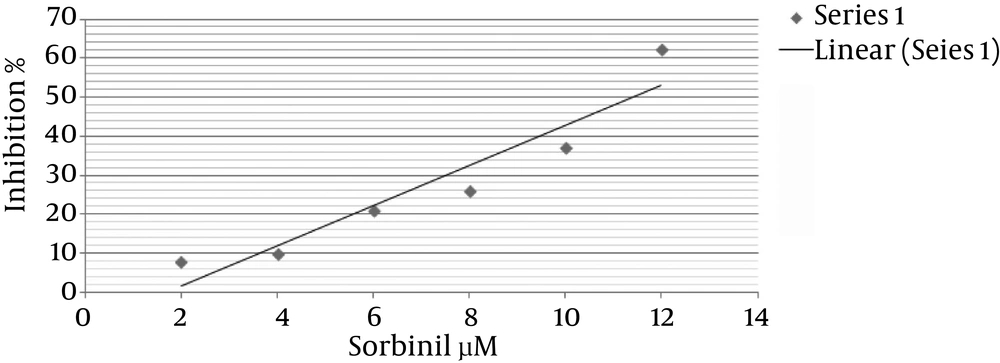
Fractions obtained from HAEP were assessed for inhibitory activity against partially purified bovine lens ALR2 in vitro (Table 1). At 1 mg/mL concentration, EThAc fraction showed the highest inhibitory activity against ALR2 with 38% inhibition. The 50% inhibition value of this fraction was measured 1.12 mg/mL (Figure 1). Sorbinil was used as positive control and standard cure of its inhibitory effect on the AR2 is presented in Figure 2.
Specificity was assessed in terms of selectivity ratio based on the 50% inhibitory capacity value of EthAc fraction of HAEP for ALR2 and then this concentration was tested to appoint the inhibition rate of ALR1 (2). It was investigated that EthAc fraction inhibited ALR2 8.25-fold more potent than ALR1, which showed its good specificity toward ALR2 over ALR1 (Table 2).
The current study investigated the effect of EthAc fraction of HAEP at ALR2 50% inhibition value on the accumulation of sorbitol in fresh human erythrocytes under high glucose condition (ex vivo), (Table 3). Sorbitol accumulation was 3.63-fold greater when RBCs were incubated in high-glucose condition (55 mM), compared to their incubation in a normal glucose condition (5.5 mM). EthAc fraction of HAEP in ALR2 50% inhibition value leads to decrease in sorbitol level in high-glucose condition amounting to 46.6%. Sorbinil was used as positive control. In negative control tube, everything was similar to test tube except that solvent was added instead of HAEP.
5. Discussion
Propolis is a traditional medicine from ancient times and has anti-cancer (29-31), free radical scavenging activity (32), anti-inflammatory (31), antimicrobial (32) and antioxidant (32) properties attributed to presence of flavonoids and phenolic compounds present in all kinds of propolis (32-35). The effect of propolis on ALR2 inhibition is not studied yet. Lens is known to have the highest ALR2 activity compared to other tissues (36); the current study investigated the effect of HAEP on bovine lens ALR2 inhibition and to test the physiological efficiency investigated the ability of HAEP to block ALR2 activity in fresh human erythrocytes, ex vivo. Among human AKRs, ALR2 is unique in this ability to catalyze the NADPH-dependent conversion of glucose to sorbitol (37) and activation of ALR2 in RBCs such as lens, retina, nerve and kidney can cause the accumulation of sorbitol (38). A study showed direct correlation between erythrocyte ALR2 activity and sorbitol level (39). The primary structure of ALR2 shows high similarities with ALR1. Both ALR1 and ALR2 are catalyzing the reduction of biogenic aldehydes and NADPH-dependent reaction of a variety of carbonyls such as glyceraldehyde, glucoronate and short-chain alkanes (25, 37). Therefore, the study also investigated the specificity of ethyl acetate fraction of HAEP with ALR1 and ALR2 and showed that HAEP is preferred for AR2 inhibition. The results of the current study showed that EthAc fraction of HAEP inhibited bovine lens AR2 with IC50 value 1.12 mg/mL. It was shown that EthAc fraction of the extract could inhibit ALR2 8.25-fold more potent than ALR1, which is good specificity compared to ALR2 over ALR1. Sorbitol accumulation was 3.63-fold greater when RBCs were incubated in high-glucose condition (55 mM), compared to their incubation in a normal glucose condition (5.5 mM). EthAc fraction of HAEP in ALR2 50% inhibition value led to decrease in sorbitol level in high-glucose condition amounting to 46.6%, therefore HAEP not only showed good and specific inhibition for AR2, but also showed inhibitory power in physiologic condition. P. Suryanarayana et al. showed that aqueous extracts of Emblica officinalis inhibited rat lens and recombinant human AR2 with IC50 values 0.72 and 0.88 mg/mL, respectively. Further, they demonstrated that the hydrolysable tannoids of E. officinalis were responsible for AR2 inhibition, as enriched tannoids of E. officinalis exhibited remarkable inhibition against both rat lens and human AR2 with IC50 of 6 and 10 μg/mL, respectively. The inhibition of AR2 by E. officinalis tannoids is 100 times higher than its aqueous extract. Activation of AR2 and increased sorbitol levels due to hyperglycemic stress were prevented when lenses were incubated with 55 mM glucose in the presence of 50 μg/mL tannoids in the medium (36). Rutin, a bioflavonoid present in many dietary sources, inhibited ALR2 with an IC50 value of 13 μM in an uncompetitive manner, but was a poor inhibitor of closely related members of the AKR superfamily, particularly ALR1. Moreover, rutin was able to suppress sorbitol accumulation in human erythrocytes under high glucose conditions, demonstrating an in vivo potential of rutin to prevent sorbitol accumulation (40). The various fractions from the methanol extract of Smilax herbacea were tested for their effects on rat lens aldose reductase in vitro. Among them, the ethyl ethanoate (EtOAc) fraction exhibited a potent rat lens aldose reductase inhibition (IC50_0.75m g/mL). Also when effective compound of this extract was administered orally at 25 mg/kg in streptozotocin (STZ)-induced diabetic rats, reduced sorbitol accumulation in the lenses, RBCs, and sciatic nerves (18). All of the above studies had IC50 value less than the current study, but it should be considered that their data were for effective compound of their extract, while the current study data were only based on raw extract. Therefore, their lowest IC50 could not necessarily represent better inhibitory activity than that of the current study extract. Indeed, a logical comparison needs more studies to recognize and utilize HAEP effective compound in another project; while the current study only meant to show the ALR2 inhibitory property of propolis, qualitatively.
5.1. Conclusion
Hydroalcholic extract of propolis is suggested as a good inhibitor for aldose reductase, in addition it has the antioxidant property and free radical scavenging activity and certain side effects that have not been reported yet. Therefore, with the possibility of mass production, it can be studied further to identify and synthesize compounds for aldose reductase inhibitions. Since the active ingredient of the current study extract was not purified and raw extract was used as inhibitor, it necessary to conduct more studies on this fraction.