1. Background
Matrix metalloproteinases (MMPs), also called matrixins, are a conserved family of zinc-dependent endopeptidases that are responsible for the hydrolysis of extracellular matrix (ECM) components (1). These metalloenzymes are either secreted or anchored in the outside cell membrane. They are involved in various physiologic processes, such as embryogenesis, organ morphogenesis, blastocyst homing, ovulation, nerve outgrowth, the endometrial cycle, angiogenesis, inflammatory cell function, bone tissue remodeling, and wound healing (2). These processes are attributed to the 25 known MMPs in vertebrates, especially 22 human homologs. MMPs determine the dynamic formation and remodeling of ECM, and therefore their activities are tightly controlled at multiple levels of production, with activation of proenzymes and inhibition by tissue inhibitors of metalloproteinases (TIMPs). Aberrant and unbalanced activities of these enzymes result in numerous pathological conditions, such as inflammation, atherosclerosis, aneurysm, arthritis, and cancer metastasis (3, 4).
MMP-9, also called gelatinase B, hydrolyzes denatured collagen and laminin, and is responsible for basement membrane degradation (5). MMP-9 is among the most important proteases involved in the development of cardiovascular disease. Migration of smooth muscle cells to the sub-endothelium, digestion of endothelial basement membrane, immune cell recruitment, restenosis following angioplasty, fibrous cap degradation of atherotic plaques resulting in thrombus formation, and establishment of vascular aneurysms are all associated with excess MMP-9 activity. Foam cell macrophages in atherosclerotic lesions are significant sources of MMP-9 secretion (6). MMP-9 is also implicated in the progression of multiple cancers and inflammatory diseases such as rheumatoid arthritis (7, 8).
Due to the essential role of MMPs in many pathologic conditions, much effort has been made to introduce efficient inhibitors against them. Tetracyclines, EDTA, hydroxamic acid derivatives (marimastat and batimastat), 1, 10-phenanthroline, and pyrimidinetrione derivatives have been studied as MMP inhibitors (9).
Tetracyclines are antimicrobial agents that have been shown to possess anti-inflammatory and MMP inhibitory effects. This effect has been studied in various models, and the inhibitory and protective effects have been reported in many cell types, animal models, and clinical trials. Based on in vitro cell culture measurements, myocardial ischemia-reperfusion rat models, and studies on patients with atherosclerotic lesions, vascular aneurysms, myocardial infarction, cerebrovascular abnormalities, periodontitis, and metabolic bone diseases, the tetracyclines (tetracycline, doxycycline, minocycline, and chemically modified tetracyclines) have shown different potencies in inhibiting various involving MMPs (10-16). In these studies, the obtained IC50 values ranged from 5 to 500 µM among the different MMPs.
2. Objectives
Considering the central role of MMP-9 in the above-mentioned diseases and the variable inhibitory potentials of routine tetracyclines, including tetracycline, doxycycline, and minocycline, this study measured the inhibitory effect of each tetracycline agent on MMP-9 activity obtained from a U-937 cell culture, a monocyte-macrophage cell line, using zymography. Investigating the inhibitory potentials of these tetracyclines against MMP-9 can demonstrate the ability and usefulness of each drug in inhibiting inflammatory processes in which this protease is involved.
3. Materials and Methods
3.1. Reagents
Electrophoresis reagents, including acrylamide, bisacrylamide, TEMED, and B-type gelatin for zymography, were purchased from Sigma (Sigma Chemical Co., St. Louis, MO, USA). Cell culture reagents, including RPMI-1640, FBS, and penicillin-streptomycin, were provided from Gibco (Invitrogen, USA). Tetracycline hydrochloride and doxycycline monohydrate were purchased from Hakim Pharmaceutical Company (Tehran, Iran), provided by Ningxia Qiyuan Pharmaceutical Co., China. Minocycline hydrochloride was purchased from Sigma.
3.2. Cell Culture
The U-937 cell line was obtained from the cell bank of the Pasteur institute of Iran. Cells were cultured according to the data sheet of the cells given by the cell bank. Briefly, the cells were centrifuged at 1,000 rpm for 5 minutes, and then resuspended in RPMI-1640 medium containing 10% FBS, 100 IU/mL of penicillin, and 100 µg/mL of streptomycin at a density of 250,000 cells/mL. The cells were maintained in a CO2 incubator with 95% air and 5% CO2 at 37°C. The cells were checked daily using an inverted microscope, to assure proper growth and lack of bacterial contamination. Healthy U-937 cells are round, relatively large, suspended cells with a clear cytoplasm. To obtain MMP-9 rich medium, the cells were scaled-up to the density of 2 × 106 cells/mL after three days of incubation. The cell supernatant was collected following centrifugation, and maintained at -80°C. The MMP-9-rich medium was used as the source of enzymes in the zymography analysis.
3.3. Preparation of Drug Dilutions
In order to draw dose-response curves for tetracyclines in MMP-9 inhibition, serial dilutions of 5, 20, 100, 200, and 500 µM of tetracycline, doxycycline, and minocycline were prepared. The doses were defined based on previous studies reporting IC50 values for MMPs. Tetracycline hydrochloride and minocycline hydrochloride are freely soluble in water. The incubation buffer of zymography was used to prepare a 50 mM solution of each drug. Serial dilutions of lower concentrations of the drugs were prepared as 100X solutions using the 50 mM concentration.
Doxycycline monohydrate is water insoluble. A mixture of 0.5 ml of HCl 0.1N and 99.5 mL of methanol was used as the solvent. A 50 mM solution was made and serially diluted to obtain other concentrations as 100X solutions.
3.4. Gelatin Zymography
Zymography gel was formed from co-polymerization of acrylamide in the presence of the MMP-9 substrate, gelatin (0.5%). Zymography is a sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis technique (17). As the source of MMP-9, 30 µL of U-937 culture medium was mixed with 5 µL of sucrose 20%, and proper volumes (30 µL) of this mixture were loaded equally into the zymography gel wells. The apparatus was set to 80 V, and electrophoresis was run for 3 hours. The gel was removed from the tank and imported in distilled water. After washing the gel two times in 2.5% triton x-100, each time for 30 minutes to exclude SDS, the gel was rinsed and placed in the zymography incubation buffer, then incubated at 37°C overnight. To study the inhibitory effects of the drugs on MMP-9 activity, 1 ml of the 100X solution of each serially diluted drug was mixed with 99 mL of incubation buffer. The zymography gels were incubated in the drug-containing incubation buffers separately. Following incubation, the gels were stained with Coomassie blue, and then de-stained with a mixture of methanol/acetic acid to reveal clear bands over a dark blue background. The optical densities of the bands corresponded to the extent of enzyme activity in each band. Densitometry of the bands was performed following gel documentation, using NIH ImageJ software, and the resulting data for the serial dilutions of different tetracyclines were used to draw dose-response curves with GraphPad Prism software. The experiments were performed in triplicate for each drug concentration. Measurements of IC50, confidence intervals, and P values of comparisons were performed with the analysis functions of GraphPad Prism software.
4. Results
4.1. Inhibitory Effects of Tetracycline on MMP-9
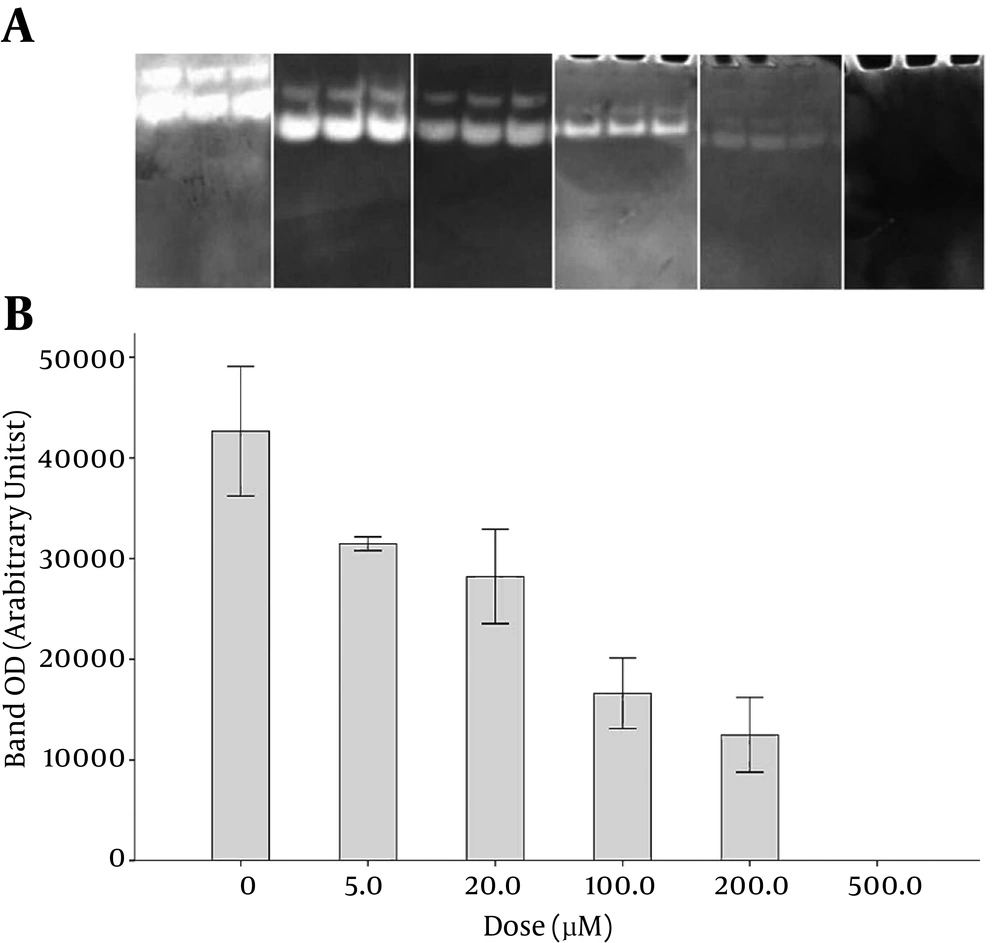
The inhibitory effects of various concentrations of tetracycline on MMP-9 activity are shown in Figure 1. As shown in the zymograms in the upper panel, increased drug concentrations resulted in lower enzyme activity and decreased optical density of the bands. At the zero concentration of tetracycline (absence of the drug), maximal activity (100%) of MMP-9 was observed, which decreased in a dose-dependent manner to 74, 66, 39, 27, and 0% in the presence of 5, 20, 100, 200, and 500 µM of tetracycline, respectively.
4.2. Inhibitory Effects of Minocycline on MMP-9
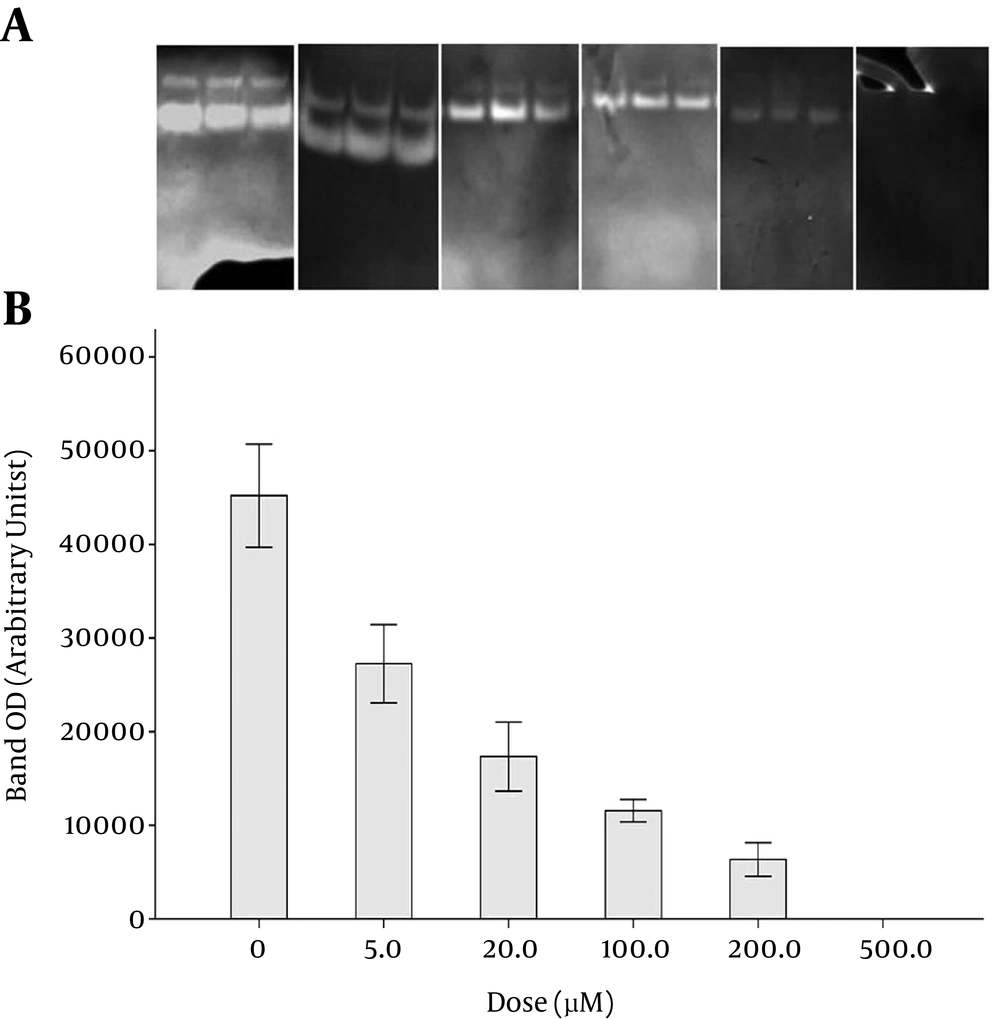
The inhibitory effects of various concentrations of minocycline on MMP-9 activity are shown in Figure 2. As shown in the zymograms, MMP-9 activity decreased dose-dependently in the presence of increasing concentrations of minocycline. The percentages of MMP-9 activity inhibition were 39, 61, 74, 86, and 100% at the concentrations of 5, 20, 100, 200, and 500 µM of minocycline, respectively.
4.3. Inhibitory Effects of Doxycycline on MMP-9
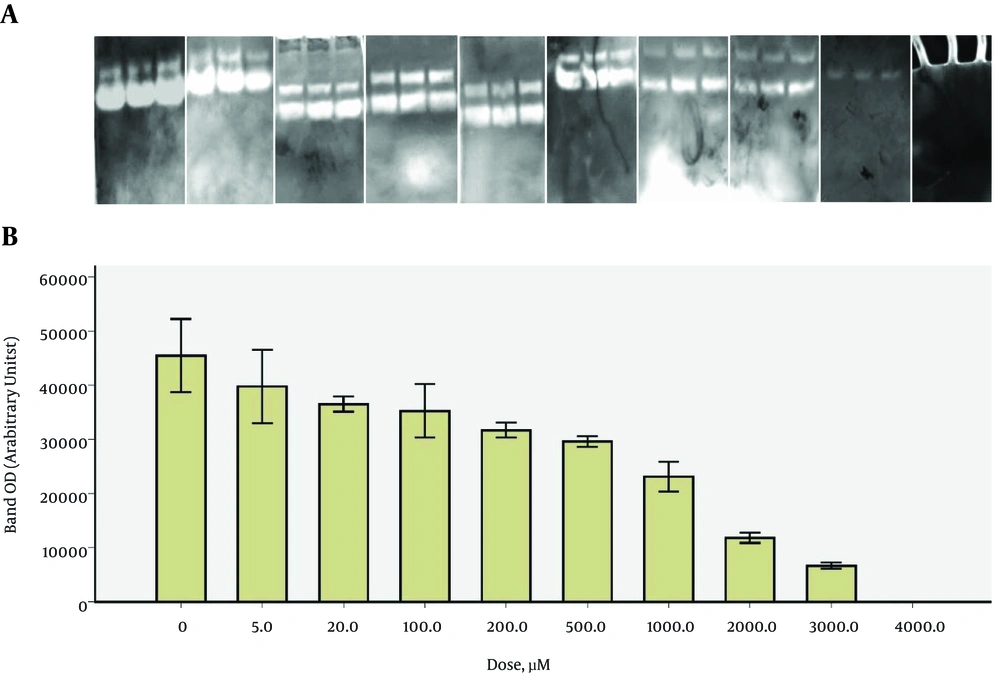
As shown in Figure 3, the optical density of the bands representing MMP-9 activity decreased with increasing concentrations of doxycycline. Complete inhibition did not occur with the dose of 500 µM of doxycycline. Higher concentrations were examined to achieve maximal inhibition. Total MMP-9 activity inhibition was seen with 4 mM doxycycline. Incubation buffers containing 5, 20, 100, 200, 500, 1,000, 2,000, 3,000, and 4,000 µM of doxycycline resulted in 13, 21, 24, 31, 36, 49, 74, 86, and 100% inhibition, respectively.
4.4. Analysis of Dose-Response Curves
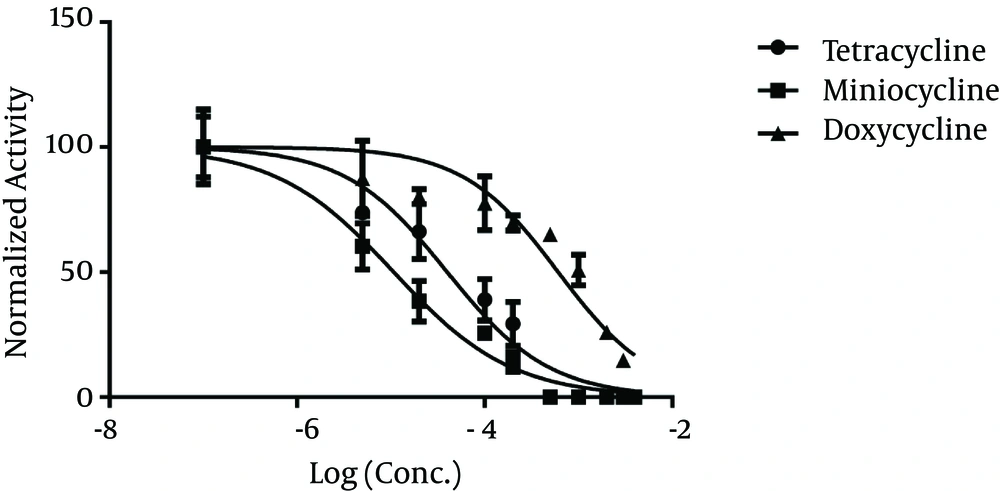
GraphPad Prism ver.6 was employed to draw dose-response curves. The densitometry data of the zymography bands that represented MMP-9 activity were drawn versus different concentrations of the drugs on a logarithmic scale to prepare the dose-response curves. The optical density of the band at zero concentration of the drugs (maximum MMP-9 activity) was considered 100% activity in the curves. Accordingly, the doses of each drug required to cause 50% inhibition in MMP-9 activity (IC50) were found to be 40.0, 10.7, and 608 µM for tetracycline, minocycline, and doxycycline, respectively.
Analysis of the curves with Prism 6 software provided confidence intervals of IC50 values of 28.4 - 56.1 µM, 7.9 - 14.5 µM, and 439.0 - 841 µM for tetracycline, minocycline, and doxycycline, respectively. Therefore, minocycline showed the highest potency in MMP-9 inhibition (P < 0.0001). Tetracycline was found to be more potent than doxycycline in MMP-9 inhibition, based on the zymography experiments (P < 0.0001).
5. Discussion
MMPs exert multiple physiologic and pathologic effects in various tissues and organs. They play the main role in maintaining the balance of dynamic changes of the ECM. Dysregulation of this balance and aberrant MMP activities result in the development of inflammation, atherosclerosis, vascular aneurysm, rheumatoid arthritis, and metastasis (2). The roles of MMPs and the effects of various MMP inhibitors have been studied in different diseases, tissues, and experimental models, including periodontitis, aneurysm, ischemia-reperfusion injury, arthritis, cancer cell metastasis, cerebral ischemia, and neurologic disease models. These studies have reported different values of efficacy in MMP inhibition. Tetracyclines, as the most common inhibitors, have shown different inhibitory and therapeutic potentials (18-22). In the current in vitro study, the inhibitory effects of tetracycline hydrochloride, doxycycline monohydrate, and minocycline hydrochloride on MMP-9 activity were investigated using zymography. Tetracyclines dose-dependently inhibited MMP-9, and IC50 values of 40.0, 10.7, and 608.0 µM were achieved for tetracycline, minocycline, and doxycycline, respectively. In previous studies on different MMPs using alternative experimental models, IC50 values ranging from 5 to 500 µM have been generally reported (13, 23-26). In a study on collagenase extracted from rabbit corneas, the inhibitory effects of tetracyclines on enzyme activity were measured using a chromogen substrate (27). In that study, the results of which conflict with ours, doxycycline showed a much lower IC50 value (15 µM) than minocycline (IC50 = 190 µM) and tetracycline (IC50 = 350 µM). In our study, minocycline showed the highest potency in MMP-9 inhibition, which is in accordance with previous reports that introduced minocycline as an effective MMP inhibitor in pathological states, such as ischemic cardiac events, cerebral neurologic damage, and arthritis (28). As a result of accumulation and concentration in cardiac cells, minocycline provides cardio protection at lower doses (29). In addition, due to its lipophilicity, minocycline can pass through the blood-brain barrier and provide neuroprotection at a high potency (low IC50) in comparison to other tetracyclines (18).
In our study, doxycycline showed a lower inhibitory effect on MMP-9 compared to tetracycline and minocycline, which is in contrast to previous findings (20, 27). The different results of IC50 values between previous studies and ours can be attributed to different pathological states, the employed experimental models, different doses and routes of administration, different tissue absorptions, the type of protease involved, and the enzyme substrate used. The effect of tetracyclines in inhibiting MMPs depends on their ability to bind Zn2+ and Ca2+, which are necessary for maintaining functional conformation and hydrolytic catalysis. Further hydrogen bonds and van der Waals interactions with the enzyme mass have been proposed, which result in disruption of the normal conformation of the enzyme (30). Tetracyclines have been shown to down regulate the expression of some MMPs, especially gelatinases. They also inhibit oxidative activation of pro-MMP zymogens (31). The association constant of tetracyclines to MMPs is not high (Kd = 70 µM), but as result of accumulation in the ECM, they can inhibit proteases. The higher the tetracycline concentration in the matrix, the more inhibition can be achieved (32).
In this study, we used the monohydrate form of doxycycline, which has much lower water solubility than tetracycline and minocycline hydrochloride. The high IC50 value of doxycycline monohydrate in MMP-9 inhibition in our work can be attributed to lower water solubility.
It has been proposed that tetracyclines’ associations with the Ca2+ and Zn2+ moieties of MMPs, and other drug-enzyme interactions, inhibit these enzymes. It is suggested that minocycline has relatively more effective interactions with MMP-9 among the studied tetracyclines.
In this study, which was an in vitro experiment, the pharmacokinetic differences of the drugs were not taken into account, and only the direct associations between drugs and enzymes determined the outcomes. Further in vitro and in vivo research focusing on the interactions of individual MMPs with tetracycline derivatives are required to establish the potent and selective inhibition of MMP-mediated disorders.