1. Background
Colorectal cancer is the second leading cause of cancer-related mortalities in developed countries. In Asian and middle eastern countries, due to changes in dietary habit and lifestyle, the incidence of colon cancer has shown a remarkable increase. Nowadays, surgery and chemotherapy are the top choices for colorectal cancer treatment and radiation therapy is the complementary therapy (1-3). However, despite all the recent developments in cancer therapy, colon cancer recurrence (50%) has remained as a major problem and it is necessary to develop some new more effective approaches for achieving a certain cell mortality rate (3, 4). Increasing the irradiation dose improves the efficiency of treatment, but the side effects of high-dose radiation including the damage of normal tissues are among the limitative factors (5). The application of radio sensitizers is one of the strategies to enhance the radiation efficiency without exceeding the maximum tolerable dose of normal tissues. Todays, gold nanoparticles (GNPs) are established as good radio sensitizers due to their high bio-compatibility and high penetration rate into cancer cells (6, 7). In addition, GNPs can be conjugated with antibodies or other proteins to target tumor cells as well as the intracellular targets such as the nucleus (8, 9). In fact, the presence of GNPs in radiation events results in local dose amplification and radiosensitization enhancement. The interactions of radiation elements with these particles lead to the production of more secondary electrons. These electrons include photo and Compton electrons with high energies. Followed by these events, a shower of Auger electrons with low energy and small range are produced. Small range of Auger electrons let them deposit their energy in the vicinity of GNPs, which leads to increased cell damage and amplified local doses (6, 10-13).
The relationship between radiation dose and radiobiological parameters can be derived with cell survival data. Linear quadratic (LQ) model is widely used to analyze cell survival data and is useful to investigate the radio resistance mechanisms and design new therapeutic trends. With this model, the radiobiologic parameters that describe the behavior of cells over the whole survival curve are accessible and also significant differences in radiosensitivity that exist among histological categories of human cell lines can be determined.
Mean inactivation dose (MID), one of radiobiological parameters, is a useful concept for the comparison of human cell survival curve. Unlike other parameters of radiobiology, MID estimates the radiation sensitivity of mammalian cells at low and high radiation doses (14). Less dependence to deviation of the survival curve of a specific cell line which was investigated by different researchers and usability for cells with different histological categories are the advantages of MID rather than other radiobiological components such as the multi-target parameters D0 and n. Accordingly, international commission on radiation units (ICRU) recommend the use of MID to characterize the cell survival curve (14, 15).
2. Objectives
Given the importance of discovering an effective method to cure colon cancer, the emergence of new models that suggest radiation dose enhancement in the presence of GNPs, and the advantages of MID as an indicative parameter of changes in cell radiosensitivity, in this study, the effect of gold nanoparticle on MID value of colon cancer cells at X-ray irradiation was investigated.
3. Materials and Methods
3.1. Cell Culture
Colorectal (HT-29) cell line was purchased from Pasteur institute (Tehran, Iran). Cells were cultured in Roswell park memorial institute (RPMI) 1640 medium (Gibco-Invitrogen), supplied by 10% fetal bovine serum (Gibco-Invitrogen), 1% antibiotic mixture containing penicillin (Sigma-Aldrich), and streptomycin (Sigma-Aldrich). The cells were stored at humidified atmosphere at 37°C with 5% CO2. The medium of cells was changed approximately every two days, and when they reached more than 80% confluency, they were split with 0.05% trypsin/0.02% EDTA and sub-cultured for more passages.
3.2. Gold Nanoparticles Cytotoxicity and MTS Assay
The cells were seeded at 2 × 103 per well of a 96-well tissue culture plate. After 48 hours, GNPs (10 and 100 µM) were added to the cells. Cell viability was investigated using MTS assay, the CellTiter 96 AQueous One Solution Cell Proliferation (ProMega), after 2, 4, 6, 24 and 48 hours. The absorbance was recorded at 490 nm in a 96-well plate reader (Biorad). Cell survival was evaluated using the following equation (Equation 1) (16).

3.3. Uptake Assay of Gold Nanoparticle
When cells reached more than 80% confluency, they were trypsinized and seeded in a 24-well plate (4000 cells/well) for 48 hours (in logarithmic phase). Consequently, the cells were exposed to 20, 40, 60, 80 and 100 µM of a 50 nm-gold nanoparticle (Sigma-Aldrich) and stored at humidified atmosphere at 37°C with 5% CO2 for 24 hours. When the incubation period passed, the culture medium was removed, the plate was washed three times with phosphate-buffered saline (PBS), the cells were counted, and 1 mL HCL (5 M) was added to each well for lysis of the cells. The concentration of GPNs was measured by graphite furnace atomic absorption spectrometry (GFAAS) analysis.
3.4. Irradiation
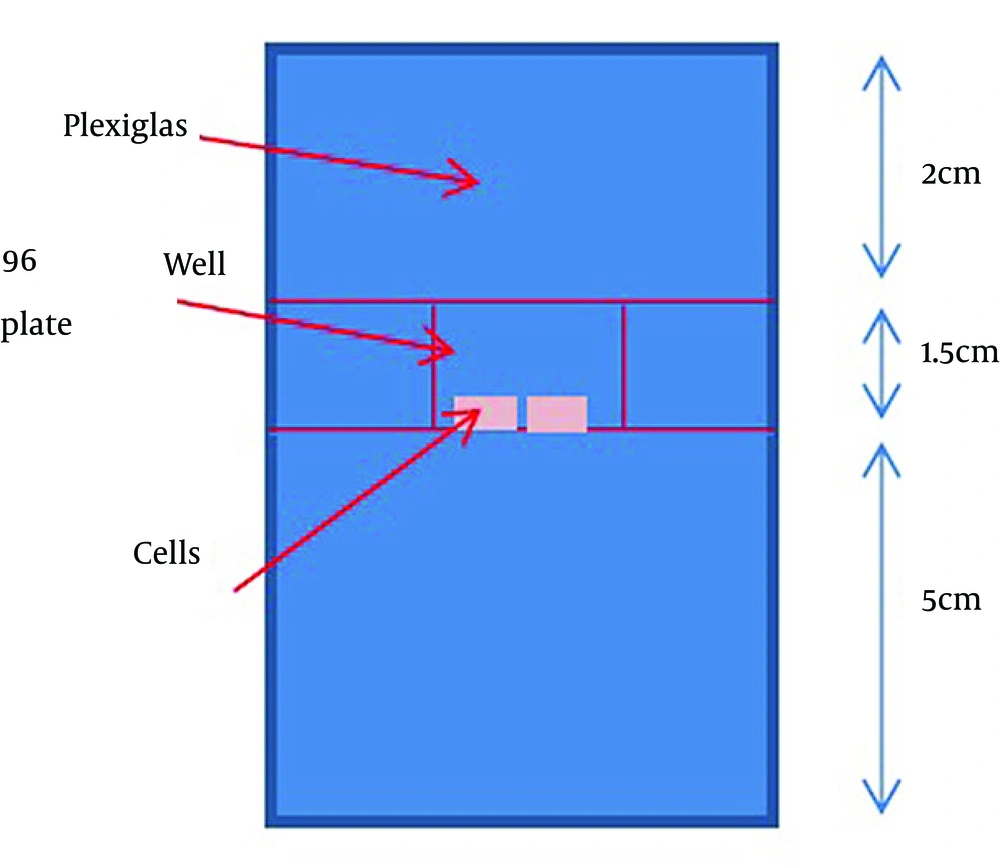
A total of 2 × 103 cells per well were plated in 96-well plates for 48 hours. Then, they were incubated with the gold nanoparticle for 24 hours before irradiation. Irradiation was performed using a clinical accelerator (Neptun 10 PC) at a source-to-surface distance (SSD) = 100 cm and 20 × 20 cm2 field size. Two centimeters of a Plexiglass sheet (water equivalent) was placed on the top of the plate to serve as a built-up material for the 9 MV beam. A Plexiglass sheet (water equivalent) of 5 cm was placed under the bottom of the plate to ensure the full backscatter condition (Figure 1). Mega-voltage radiation (9 MV) was delivered at total doses of 2, 4, 6 and 8 Gy with a dose rate of 300 cGy min-1. In vivo radiation diode dosimetry measurements were performed for beam calibration and variation within a field was smaller than 2% for each well. After irradiation, the cells were incubated at 37°C with 5% CO2. All the treatments were carried out in triplicate and were repeated.
3.5. Survival Fraction-Based Multiple-MTS Assay
Multiple-MTS assay was used to estimate the survival fraction (17). In multiple-MTS assay, the cell growth data (the number of viable cells) are collected daily to draw the cell growth curve. The survival fraction can be estimated by measuring the time difference between the cell growth of the control and the treated groups to reach a certain value. Using the CellTiter 96 AQueous One Solution Cell Proliferation Assay (ProMega), cell viability tests were performed directly after irradiation (1 - 7 days) and cell growth curve was obtained.
Survival fraction was calculated by the following equation (Equation 2) (17):

Where t doubling time is the period required for a quantity of cells to double and t delay is the period to reach the specific absorption value of control versus irradiated cells.
3.6. Evaluation of Mean Inactivation Dose and Radio Sensitization Enhancement in the Presence of Gold Nanoparticle
MID is equal to the area under the cell survival curve. After estimating the survival fraction at different radiation doses, the survival curve (survival fraction versus radiation dose) should be fitted to the linear quadric model (Equation 3). MID was calculated using the following equation (Equation 4) (14).


The Sensitizer enhancement ratio (SER) value was calculated by dividing the MID of cells not exposed to GNPs to cells exposed to GNPs.
4. Results
4.1. Gold Nanoparticles Uptake in HT-29 Cells
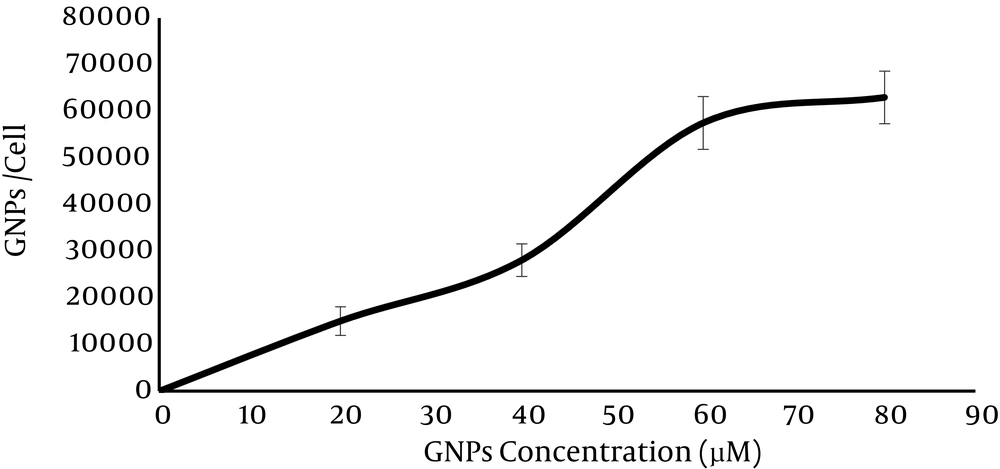
HT-29 was incubated with GNPs in varying concentration (20, 40, 60, 80 and 100 µM). Figure 2 shows the average gold nanoparticle uptake by each cell in an individual concentration. It indicates that the uptake of GNPs increases with increase in GNPs concentration. The maximum uptake was observed in 80 µM (62.98 ± 3.49) × 103 and there was no significant difference in GNPs uptake between 80 µM and 100 µM (P-value = 0.1 > 0.05).
4.2. Cytotoxicity of Gold Nanoparticles
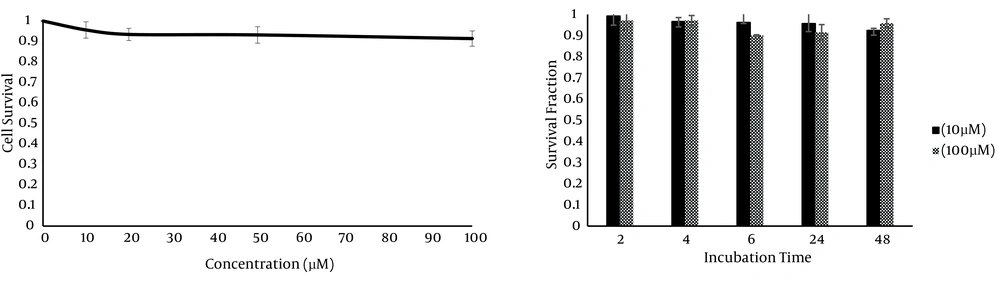
To determine the cytotoxicity of GNPs on HT-29 cells, the cells were incubated with different concentrations of GNPs (10, 20, 50 and 100 µM) for 24 hours. The cytotoxicity of GNPs at different concentrations was measured by MTS assay. Figure 3 a shows no significant difference between the controls and those treated with the GNPs over the whole range of GNP concentrations (P value > 0.05). In addition, because the cytotoxicity of nanoparticles could be time-dependent, MTS assay was performed at different incubation times (2, 4, 6, 24 and 48 hours). Cell viability at different times was over 90% in all the groups (P > 0.05) (Figure 3 b). Collectively, this data indicated that GNPs did not have remarkable cytotoxicity on HT-29 cells in all the studied concentrations (10 - 100 µM).
4.3. Cell Survival Fraction Curve in the Presence of Gold Nanoparticles
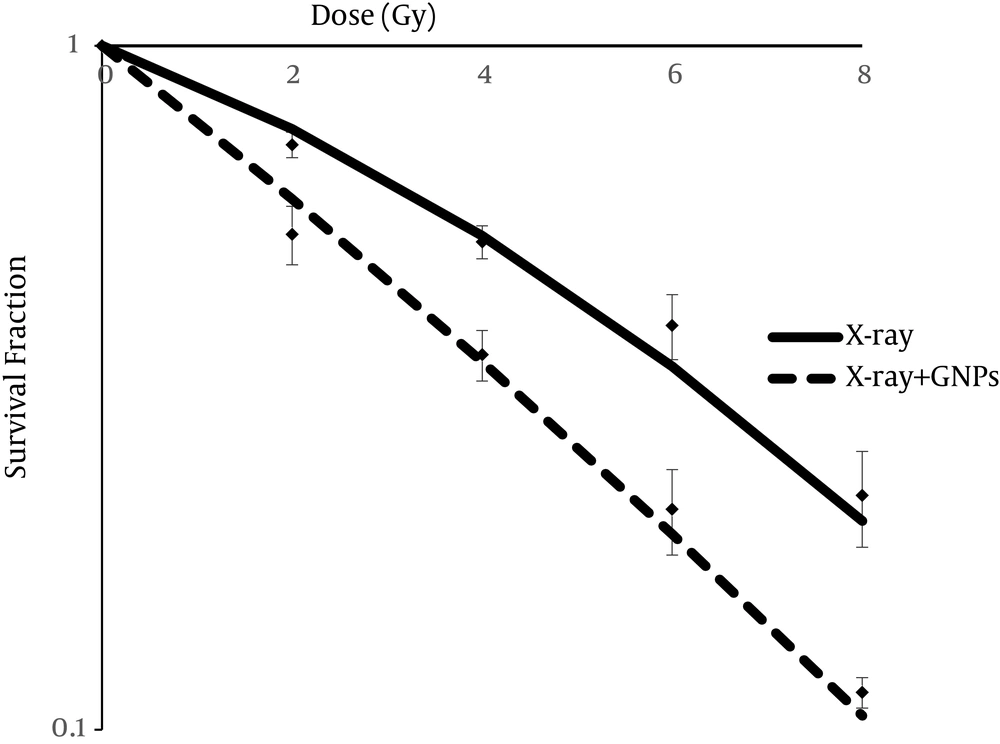
For evaluating the effectiveness of GNPs on radiosensitivity of HT-29 cells, they were incubated with a 60 µM concentration of GNPs for 24 hours and then were expose to 2, 4, 6 and 8 Gy of 9 MV X-ray energy. Figure 4 Shows that GNP-irradiated cells decreased more than alone-irradiated cells. Multiple-MTS assay was used to quantify the survival fraction of irradiated cells in the presence and absence of GNPs. MTS assay was conducted in several days and cell growth curve was drawn for control, GNPs alone, X-ray alone, and X-ray with GNPs groups. The survival fraction of alone irradiated cells and GNPs-irradiated cells was estimated based on the delay that occurred the between growth curve of the control and treated cells. Figure 5 shows the survival curve of the two mentioned groups based on the LQ-model. As can be noted from Figure 5, radiation combined with GNPs induced greater decreases in survival fraction at different radiation doses (P < 0.05).
4.4. Effect of Gold Nanoparticles on Mean Inactivation Dose
After drawing the cell survival curve, MID value was estimated using Equation 4. The MID value in cells only irradiated was 5.03 and significantly reduced in cells irradiated in the presence of GNPs (3.61).
Sensitive enhancement ratio (SER) that was calculated by dividing the MID value to the two mentioned groups, was 1.4.
5. Discussion
Cell radiosensitivity can be predicted by radiobiological parameters extracted from the survival curve. Mean inactivation dose is one of these parameters that is introduced for characterizing of cell radiosensitivity over the whole survival curve with a single parameter. The usability for the whole cell population and the presentation of the consistency results make this parameter more acceptable for describing cell radio sensitivity compared to other sets of radiobiological parameters (14, 18, 19).
In several theoretical and experimental studies, using GNPs as radiosensitizers at orthovoltage (kV) and megavoltage (MV) energies has been demonstrated by amplifying local radiation dose and consequently increasing the rate of cell death (20-22). Recently, researchers reported that GNPs can enhance the radiosensitization of human carcinoma cells such as prostate, ovarian and breast cancer cells. Jain et al. (10) demonstrated that GNPs radiosensitization depended on the type of cell. Our results indicated that GNPs irradiation induced greater decreases in survival fraction than alone irradiation at different radiation doses and enhanced the radiosensitization of colon cancer cells. Based on previous studies and given the importance of MID parameter to predict radiosensitization, the present study aimed to calculate the SER of colon cancer cells by dividing MID of cells not exposed to GNPs to cells exposed to GNPs.
The cytotoxicity and the uptake of GNPs are two important parameters that influence radiosensitization (12, 23). Our results clearly revealed that cell viability was more than 90% when colon cancer cell (HT-29) was incubated with GNPs at 10 - 100 µM. Based on the steps of the uptake assay, GNPs on the cell surface were removed after three times washing with PBS and the average numbers of naked-GNPs uptake in 60 µM and 80 µM were (5.749 ± 5.65) ×104 and (6.298 ± 3.49) × 104, respectively, and there was no significant difference in uptake (P = 0.09 > 0.05). Wang et al. (24) showed that the amount of Glu-GNPs in A549 cells was approximately 12 × 104 and demonstrated that significant radiosensitization occurred in A549 cells with an SER of 1.49 at 6 MV X-rays with Glu-GNPs. In another study on MDA-MB-231 cells (10), the SER was found to be 1.29 and 1.16 for 6 MV and 15 MV irradiations, respectively, and in a similar study (25), the corresponding values were 1.24 ± 0.05 and 1.18 ± 0.04. Taken together these data, determining SER-based MID value is a useful approach for predicting the radiosensitizing effects of GNPs. In agreement with previous studies, the obtained results revealed a significant reduction in survival fraction of HT-29 cells that were exposed to 9 MV X-ray with GNPs over the whole survival curve (P < 0.05). The MID value of HT-29 cells that were incubated with GNPs (3.61) significantly decreased rather than the ones not exposed to GNPs (5.032) and the SER was 1.41.
In conclusion, our results suggested that in the presence of GNPs, the MID value of HT-29 cells reduced and consequently GNPs can induce radiosensitization enhancement in colon cancer cells.