1. Background
One of the most common types of headache is migraine that often begins before 30 years age. One-third of migraine sufferers usually have three or more attacks per month. These patients need to bed rest and their daily activities limit, so the prophylactic treatment is very important (1, 2). The important classes of drugs for migraine prophylaxis are beta-blocker, calcium channel blockers, tricyclic antidepressants and anticonvulsants. Beta-blockers interact with serotonin receptors. Calcium channel blockers prevent intracellular calcium entry. Tricyclic antidepressants act as reuptake of serotonin inhibitors at central sites (2-4). The drugs for migraine prophylaxis are not completely effective or safe (3). The use of complementary preventive treatments has been growing worldwide that can be decrease the frequency and severity of migraine attacks with minimal side effects (5, 6).
Tanacetum parthenium L. (common name: feverfew) from Compositae family is a native of Europe’s Balkan mountains and now grows worldwide (North and South America, Europe, North Africa, Asia and Australia). Feverfew is a perennial plant that grows 15 - 60 cm tall with small flowers and yellow-green leaves (7). The leaves are used as medicinal parts that have traditionally been used for the treatment of arthritis, toothache, asthma and stomach-ache. Also, feverfew has been used as a febrifuge and remedy for migraine prophylaxis (8). The results of clinical studies showed feverfew has anti inflammatory, anti nausea and vomiting effects. Furthermore it can be used as promoting restful sleep and improving digestion (9). There are a number of clinical trials demonstrated efficacy and safety of feverfew in migraine prophylaxis. Studies showed a good effect of feverfew in the prophylactic treatment of migraine while there is no report of serious side effects in clinical trials (10-12). According to previous investigations the active compounds of feverfew are sesquiterpene lactones principally parthenolide. The extract of feverfew and pure parthenolide inhibit serotonin release from blood platelets and also they inhibit the synthesis of prostaglandins (13).
2. Objectives
In present study, the effect of feverfew cultivated in Iran has been studied as a prophylactic treatment of migraine in a placebo-controlled clinical trial. T. parthenium has been recently cultivated by Zardband Botanical garden in Iran for pharmaceutical purposes. In this study, participants referred to Golestan hospital, Ahvaz, Khouzestan province, Iran.
3. Patients and Methods
3.1. Plant Material
The aerial parts of T. parthenium were collected from Zardband Botanical garden, Iran.
The aerial parts were aired dried in shade and extracted with ethanol/water (80/20) at room temperature, for 72 hours consequently. The extract was dried using rotary evaporator and then stored at 4°C until required. The parthenolide content of the aerial parts was 0.4% as determined by HPLC. The drug is standardized based on parthenolide. The extract was supplied in bottles of 20 ml. Placebo was prepared in same physical form, packaging and labeling as feverfew extract. The approved additives and color were added to the placebo. The bottles of the drug and placebo divided to groups 1 and 2 and were used for patients.
3.2. Patients
Criteria for inclusion were migraine without aura diagnosed after a careful medical history-taking according to the International Headache Society (IHS) criteria (14). Sixty patients (45 women and 15 men) with range 15 to 50 years old, who attended the Golestan hospital, Ahvaz, Khouzestan province, Iran were included in the study. The patients with 2 - 8 migraine attacks per month for at least one year were enrolled in this study. Exclusion criteria included: age < 15 or > 50, other kinds of headache, secondary headache (tumor, fever, meningismus, loss of consciousness, focal neurological symptoms), pregnancy, breastfeeding, use of other medicinal herbs and loss of using the drug for more than 3 days.
3.3. Design
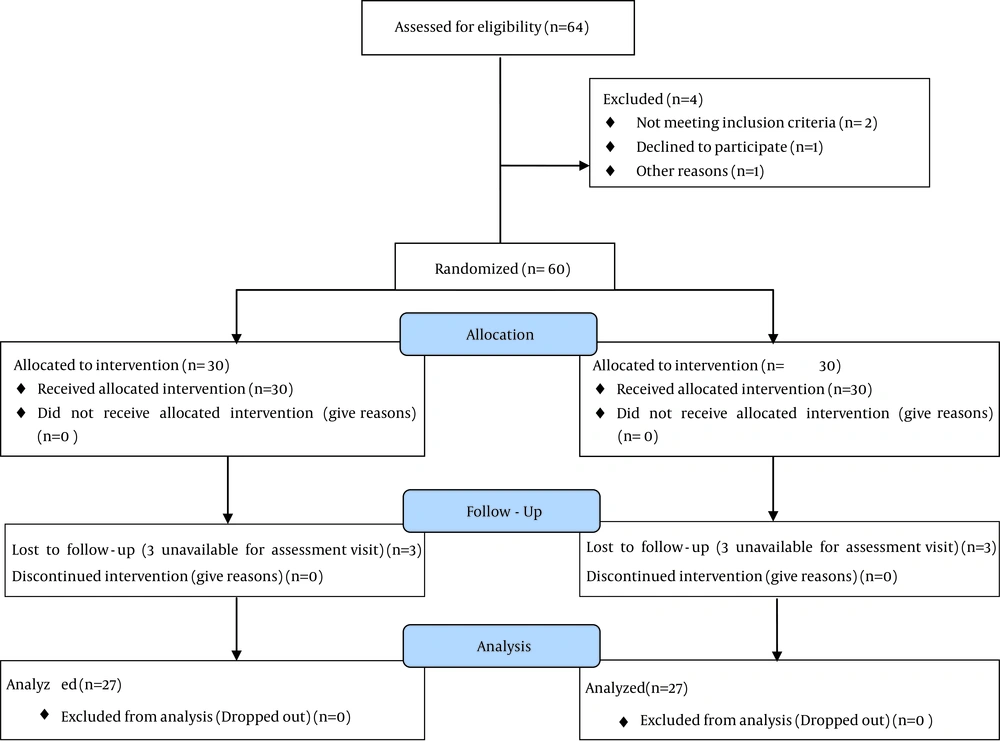
The present study was designed as a double-blind randomized controlled clinical trial. The present clinical trial was approved by Ahvaz Jundishapur University of Medical Sciences Ethics Committee. The participants were divided at random into two groups (1 and 2). Random group assignments were done using a simple random allocation strategy, using a block randomization method. The Study flowchart is shown in figure 1. Both groups received tablet propranolol 40 mg per day and 10 drops of drug (parthenolide 0.4%) or placebo every night for three months as a complementary preventive treatment. The patients used the drug or placebo after dinner and 30 minutes before bedtime. The Migraine Disability Assessment Scores (MIDAS) questionnaire is quite a useful tool in clinical trial that the patients were recognized for migraine impact at the baseline and end of the study (15). There were 5 questions that asked patients relating to their disability associated with their migraine headache during the last three months. The results of the MIDAS show a total score reflecting the degree of disability in patients, the score of 0 to 5 (days) shows little or no disability, score from 6 to 10 indicates mild disability, 11 to 20 moderate disability and 21 or more, severe disability. Furthermore, there were two questions that indicated the number of headache days and headache intensity during the previous 3 months. The patients described headache severity between 0 - 10 where score 10 showed the maximum intensity headache.
The occurrence of side effects was investigated at visits. The patients were evaluated about gastrointestinal disorder, diarrhea, nausea, vomiting, abdominal pain, dizziness, depression, back pain, pruritus, erythematous rash, increased sweating, paraesthesia, anorexia, insomnia, nervousness, dry mouth, gastroenteritis and menstrual disorder.
3.4. Data Analysis
Sample size calculations were based on the previous studies (12). The data are expressed as mean ± standard deviation. For each groups changes in the MIDAS score, frequency and intensity of headache was compared by paired-sample t - test at the beginning of the study and then at the end of the study. Statistical significance was considered at P < 0.05.
4. Results
In the present clinical trial, the effect of feverfew as a prophylactic therapy for migraine was studied. The information of the study population is shown in Table 1. In this study, there were sixty patients (45 women (75%) and 15 men (25%)) that randomly assigned to receive drug or placebo. In the treatment group, 22 women and 8 men aged 29.36 ± 7.70 and in placebo group 23 women and 7 men aged 28.06 ± 7.04 were enrolled. There were no significant differences between patients in terms of age or gender in treatment and placebo groups (P > 0.05). Out of the 30 patients in treatment group three cases were excluded when they were unavailable for assessment visit. Three patients discontinued treatment in placebo group when they were unavailable for assessment visit. The remaining 54 patients successfully completed the 3-month clinical trial for prophylactic therapy of migraine with feverfew and were included for analysis.
a Data are presented as mean ± SD.
The MIDAS scores in the treatment and placebo groups at the beginning of the study were 42.37 ± 7.62 and 41.33 ± 11.74, respectively that the MIDAS scores in two groups was not significant (P = 0.70). In the case group, after three months of treatment with feverfew, the MIDAS score was reduced to 19.18 ± 4.02. During treatment, participates did not report any complaints and side effects. In the placebo group, at the end of study, the MIDAS score was reduced to 29.18±9.62. Compared to placebo group, the changes in the MIDAS score, in the treatment group was significant, at the end of the study (P = 0.001). The reduction of 54.73% in MIDAS score over three months attests to the clinical significance of feverfew effect. The reduction in the MIDAS score during treatment was significant for both groups (paired-sample t-test: feverfew group P = 0.00, placebo group P = 0.00). The two groups (placebo and drug) did not differ significantly in the frequency and severity of migraine before treatment with feverfew or placebo (P > 0.05). Patients in the case group claimed that they had suffered 7.14 ± 1.17 attacks each month before treatment with feverfew and that during treatment the monthly rate had fallen to 2.74 ± 1.34 attacks. Patients in the placebo group had suffered 7.22 ± 1.05 attacks each month at the baseline of study and 4.15 ± 1.10 at the end of study. The reduction in the frequency of migraine during treatment was significant for both groups (paired-sample t-test: feverfew group p = 0.001, placebo group p = 0.001). In the treatment group the severity of headache at the beginning of study, was 7.19 ± 0.92 and at the end of study was 3.15 ± 1.06, that showed the significant reduction (P < 0.05), (Table 2). In the placebo group the severity of migraine in the baseline and end of study were 7.00 ± 1.27 and 4.67 ± 0.92 that showed the significant reduction (P < 0.05), (Table 2). Thus when the two groups were recruited to the study their frequency and severity of migraine each month were similar. Compared to control group, the changes in intensity and frequency of migraine, in the treatment group was significant, at the end of the study (P < 0.05). The frequency and severity of migraine in case group after one month of feverfew therapy are 3.85 ± 1.59 and 4.18 ± 1.11, respectively that showed the significant reductions (P < 0.05).
| Variable | Treatment Group | Control Group | P Value |
|---|---|---|---|
| MIDAS scores | |||
| Baseline | 42.37 ± 7.62 | 41.33 ± 11.74 | 0.70 |
| 1 month after feverfew therapy | - | - | - |
| 3 months after feverfew therapy | 19.18 ± 4.02 | 29.18 ± 9.62 | 0.001 |
| No. of headache days per month | |||
| Baseline | 7.15 ± 1.17 | 7.22 ± 1.05 | 0.80 |
| 1 month after feverfew therapy | 3.85 ± 1.58 | 5.00 ± 1.35 | 0.006 |
| 3 months after feverfew therapy | 2.74 ± 1.34 | 4.14 ± 1.23 | 0.001 |
| Headache severity | |||
| Baseline | 7.18 ± 0.92 | 7.00 ± 1.27 | 0.54 |
| 1 month after feverfew therapy | 4.18 ± 1.11 | 5.18 ± 1.35 | 0.005 |
| 3 months after feverfew therapy | 3.14 ± 1.06 | 4.66 ± 0.91 | 0.001 |
a Data are presented as mean ± SD.
5. Discussion
In current study, efficacy of feverfew migraine prophylaxis in a randomized, double-blind, placebo-controlled was investigated of 60 migraine patients. From the analysis of the efficacy criteria, the MIDAS score, migraine intensity and frequency were reduced by extract of T. parthenium.
Of the 27 patients who completed the study, there was a 56.26% and 61.62% reduction in severity and frequency of migraine attacks during feverfew treatment, respectively. Regarding the safety of feverfew extract the incidence of side effects in the treatment group was similar to placebo. The effective dose for migraine headaches is 100 – 300 mg, standardized to contain 0.2 – 0.4% parthenolides (16). Canada's Health Protection Branch granted a Drug Identification Number (DIN) recommends 125 mg per day of dried feverfew leaf containing at least parthenolide 0.2% for the prophylactic treatment of migraine (17). In this study, the parthenolide content of the Iran cultivated feverfew was 0.4% that for migraine prevention is necessary.
Some studies showed feverfew might inhibit cytochrome P450 1A2 (CYP1A2) and increase the levels of drugs metabolized by CYP1A2 in humans. In present study, the patients used propranolol that metabolized by CYP450, so the level of propranolol might increase (18, 19). Some of the observed improvement in patient headache may be due to the fact that propranolol serum level was higher in the feverfew group.
In some previous clinical trials, the effect of feverfew as a migraine prophylaxis was studied.(10, 11) In one study in Germany, the results indicated that the extract of feverfew with a dosage of three capsules of 6.25 mg per day reduced the migraine attacks per month (11). In a clinical trial, 72 migraine patients received dried feverfew leaves (82 mg) or placebo per day for four months. The frequency of migraine attacks in feverfew group was reduced 24%, furthermore nausea and vomiting in the migraine attacks was reduced, significantly (10). In the other double-blind clinical trial, 17 migraine patients received feverfew leaves (50 mg/day) or placebo for 6 months. The frequency and severity of headache, nausea and vomiting in placebo group was increased while there was no change in the migraine frequency and intensity in feverfew group (12). In a randomized clinical trial, the effect of a combination of riboflavin, magnesium and feverfew for prevention of migraine was investigated. The treatment group received drugs for three months. The results indicated a significantly reduction in frequency of migraine attacks but riboflavin 25 mg showed more effect than combination therapy (20). The combination of feverfew and Salix alba was used for migraine prophylaxis for twelve weeks. The frequency and severity of migraine were reduced 61.7% and 62.6% at 12 weeks, respectively (21). In present study, frequency and severity headache were reduced more than 50%.
Different limitations may have affected the findings of the study such as, the bitter taste of drug and low compliance by patients, small sample size and the positive effect of propranolol.
In conclusion, the extract of feverfew cultivated in Iran showed a significant migraine prophylactic effect with no adverse events. Accordingly, in a current clinical trial this effect is studied in 60 migraine patients with 2 - 8 migraine attacks per month for at least one year. In this study, patients used propranolol and some of the prophylactic effect may be due to the propranolol. The authors suggested to evaluation of feverfew without using propranolol. Because of the low number of patients, these findings need to be confirmed in a larger randomized controlled investigation. Because of the bad taste of feverfew drop is proposed to study the effects of feverfew in other dosage forms like capsule.