1. Background
A main strategy in the development of potential anti-cancer drugs is to identify and characterize novel agents that can regulate apoptosis-signaling pathways (1). The wide diversity of plant-derived bioactive compounds makes them popular candidates as drugs for potential use as cancer therapeutics. In this regard, several studies have identified several plants and phytochemicals that impact many molecular pathways leading to programmed cell death (2).
The genus Vincetoxicum Wolf (family Apocynaceae, including Asclepiadaceae), with about 70 different species, is widely present in many areas of the Caucasus range and in China. Seven species are known to be present in Iran and two are endemic: V. mozaffarianii Zaeifi and V. assadi Zaeifi (3-5). Among the Vincetoxicum species, a few are known to impart biological effects (6). For example, V. rossicum (Kleopow) Barbar contains potent inhibitors of pathogenic fungi and bacteria (7). The n-hexane and ethyl acetate fractions of V. stocksii Ali and Khatoon were seen to exhibit anti-leishmanial activity against Leishmania donovani promastigotes (8, 9). These extracts were also found to be active against Candida albicans, Bacillus subtilis, and B. cereus (10). A crude extract of V. stocksii was found to combat hyperactive states of the gut (9). Additionally, constituents in V. lutea L. were shown to possess anti-oxidant and radical scavenging activities (11).
While it is interesting to note these overall biologic effects of various Vincetoxicum species, toxicology/pharmacology studies require identification of the agents within these natural products that are the active components. In that regard, glycosides of V. hirundinaria Medik. (12), flavonoid heterosides of V. officinale Moench (13), heterosides of quercetin from V. officinale (14), phenanthroindolizidine alkaloids from V. pumilum (15), and phenanthroindolizidine alkaloids and N-oxide alkaloids from V. hirundinaria Medik. (16) are among the multiple classes of compounds associated with species of the genus Vincetoxicum. Some of these agents have been thought responsible for the observed cytotoxic properties. For example, in vitro studies revealed that both phenanthroindolizidine alkaloids and N-oxide alkaloids from V. hirundinaria (Cynanchum vincetoxicum Pers.) were responsible for the cytotoxic effects observed against cancer cells (16, 17).
2. Objectives
Based on the lack of knowledge about the biologic effects of V. pumilum, this study was designed to explore the cytotoxic effects of V. pumilum against HL-60 and K562 human leukemia cell lines, and to ascertain if apoptosis could be a mechanism underlying any observed toxicities. In this way, we might be able to provide initial evidence to support our hypothesis that this natural product could eventually have a potential useful role in the treatment of some types of cancers.
3. Materials and Methods
3.1. Reagents and Chemicals
AlamarBlue® (resazurin) was purchased from Sigma (St. Louis, MO, USA). RPMI 1640 and fetal calf serum (FCS) were obtained from Gibco (London, UK). Lympholyte®-H was bought from Cedarlane (Montreal, Canada). Antibodies to β-actin and PARP, along with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG, were purchased from CellSignaling Technology (Boston, MA, USA). ECL western blotting detection reagent was obtained from BioRad (Hercules, CA, USA). Propidium iodide (PI), protease inhibitor cocktail, phosphatase inhibitor cocktail, sodium citrate, Triton X-100, phenylmethylsulfonyl fluoride (PMSF), and a QuantiPro BCA RPMI-1 Assay Kit were purchased from Sigma (Steinheim, Germany).
3.2. Plant Materials
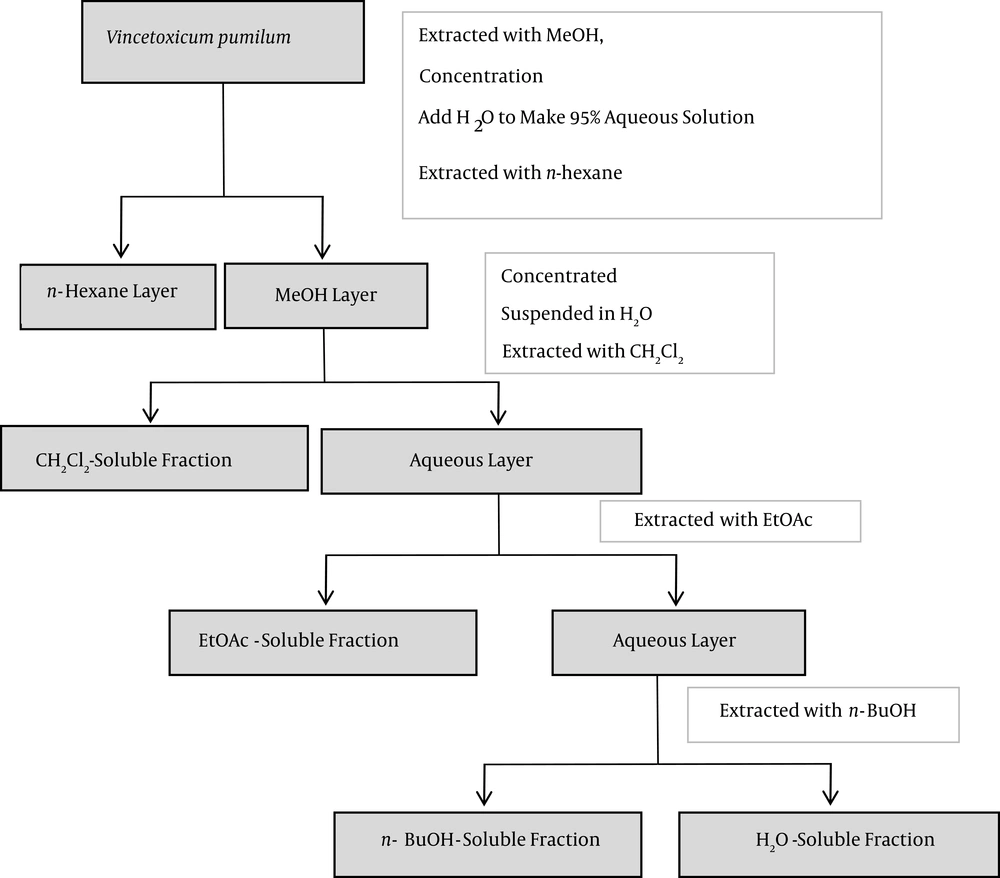
The roots of V. pumilum were collected in July 2010 from Deh-Gheibi (990 m above sea level), a village near Mashhad, Razavi Khorasan province, in northeast Iran. The plant was verified by Mrs. M. Souzani at the herbarium of the school of pharmacy (Mashhad University of Medical Sciences), where a voucher specimen (No. 12753) was deposited. A sample of the roots was dried and powdered, and ~ 225 g of the material was ‘cooked’ in 100% methanol (MeOH) at room temperature. The extract was then filtered and the solvent was evaporated under reduced pressure at 40°C - 45°C to yield a crude methanol extract (39.02 g). A portion of the extract (30.77 g) was then re-suspended in 95% methanol and partitioned successively between n-hexane, methylene chloride (CH2Cl2), ethyl acetate (EtOAc), n-butanol (n-BuOH), and finally water, based on increasing solvent polarity. The partitioning scheme for the V. pumilum methanol extract is presented in Figure 1. The n-hexane, CH2Cl2, n-BuOH, and EtOAc fractions were each then evaporated under vacuum to yield residues of 1.91, 2.93, 0.53, and 0.74 g, respectively. The water fraction was freeze-dried. All final extracts were stored at -20°C until analyzed or used in the studies outlined below. Each isolate was dissolved in dimethyl sulfoxide (DMSO) when needed for use in those assays.
3.3. Cell Culture
Human leukemic cancer cells HL-60 and K562 were obtained from the Pasteur Institute (Tehran, Iran). Peripheral blood lymphocytes were collected from a healthy donor. The study protocol to harvest the peripheral blood lymphocytes was approved by the ethics committee of the Mashhad University of Medical Sciences. In all cases, cells were maintained in RPMI-1640 medium containing 10% FCS, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
3.4. Cell Viability
A resazurin reduction assay (18) was performed to assess the toxicity of the methanol extract and the n-hexane, CH2Cl2, EtOAc, n-BuOH, and H2O fractions of V. pumilum toward the HL-60 and K562 human leukemic cancer cell lines, as well as the isolated human blood lymphocytes. Resazurin (the active ingredient in alamarBlue® reagent) is a permeable agent that, upon entering cells, is reduced to highly fluorescent resorufin. Viable cells continuously convert resazurin to resorufin, increasing the overall fluorescence.
In this study, 5 × 104 cells were placed in each well of a 96-well cell-culture plate in a total volume of 100 µL. Thereafter, varying concentrations of the extract or different fractions were added to dedicated wells in 100 µL aliquots. After 48 hours, 20 µL of resazurin (0.01% w/v in H2O) was added to each well, and the plates were incubated at 37°C for 4 hours before the absorbance was measured at 540 nm (test wavelength) and 630 nm (reference wavelength) in a Synergy H4 Hybrid Multi-Mode Microplate Reader (BioTek, Winooski, VT, USA). The cytotoxicity of the extract and fractions was expressed as IC50, calculated using Prism 5 Software (GraphPad, La Jolla, CA, USA), and presented as mean ± SEM from three independent experiments (with three replicates for each concentration tested [extract or fraction]). For each study, a control sample remained untreated and received only medium in place of the test materials.
3.5. Cell Morphology
The HL-60 and K562 cells were placed in 96-well plates at a density of 5 × 104 cells/well, then varying concentrations of the methanol extract or different fractions (3.9 - 250 µg/mL) were added as described above. The cells were grown at 37°C for 48 hours before the culture plates were examined and photographed using an inverted light microscope.
3.6. PI Staining
Any appearance of a so-called sub-G1 peak in the flow cytometry histograms of the treated cells can be detected using PI staining (19, 20). Briefly, 5 × 105 cells were seeded into the wells of a 24-well plate and treated for 48 hours with different concentrations (0.0, 1.25, 2.5, 5.0, and 10.0 µg/ml) of the CH2Cl2 fraction of V. pumilum. This particular fraction was selected for analysis because it displayed the highest cytotoxic activity in test cell lines in pilot studies.
Floating and adherent cells were then harvested and incubated at 4°C overnight in the dark with 750 μL of a hypotonic buffer (50 μg/mL PI in 0.1% sodium citrate + 0.1% Triton X-100), before flow cytometric analysis using a FACScan™ flow cytometer (Becton Dickinson, San Diego, CA, USA) was performed. A minimum of 10,000 events was acquired for each sample. All data were then analyzed using WinMDI version 2.8 software.
3.7. Western Blot Analysis
Samples of 5 × 106 K562 and HL-60 cells each were seeded into dedicated wells of a 6-well plate and treated with V. pumilum methanol extract (50 μg/mL) or its CH2Cl2 fraction (1 or 2 μg/mL) for 48 hours. The cells were then rinsed with cold phosphate-buffered saline (PBS, pH 7.4) three times, and the final cell pellet was re-suspended in lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% Triton-X 100, 1 mM EDTA, 0.2% SDS, 1% protease inhibitor cocktail, 1% phosphatase inhibitor cocktail, and 1 mM PMSF) and left on ice for 30 minutes. Thereafter, the sample was centrifuged (10,000 rpm, 20 minutes, 4°C) and the cell lysate collected. After determining the protein concentration (BCA assay), equal amounts of lysate proteins (100 µg/lane) were subjected to electrophoresis over a 12.5% SDS-PAGE gel. After resolution, all proteins were electrotransferred to a polyvinylidene fluoride (PVDF) membrane and subjected to immunoblotting using rabbit anti-β-actin, -PARP, or -Bax primary antibodies (each at 1: 1000 dilution). After 12 hours of incubation at 4°C, the membranes were rinsed with PBS and then treated with HRP-conjugated anti-rabbit IgG secondary antibody (1: 2000 dilution) for 2 hours at room temperature. The levels of β-actin, enhanced Bax protein, and PARP cleavage products in each lysate were then detected using enhanced chemiluminescence afforded by the ECL Western blotting detection reagent. Images were then quantified using a Model GS-800™ Calibrated Imaging Densitometer (Bio-Rad).
3.8. Statistics
One-way analysis of variance (ANOVA) and a Bonferroni’s post-hoc test were used for data analysis. All results were expressed as mean ± SEM. A P ≤ 0.05 was considered statistically significant.
4. Results
4.1. Constituent Presence in Methanolic Extract and the Various Sub-Fractions
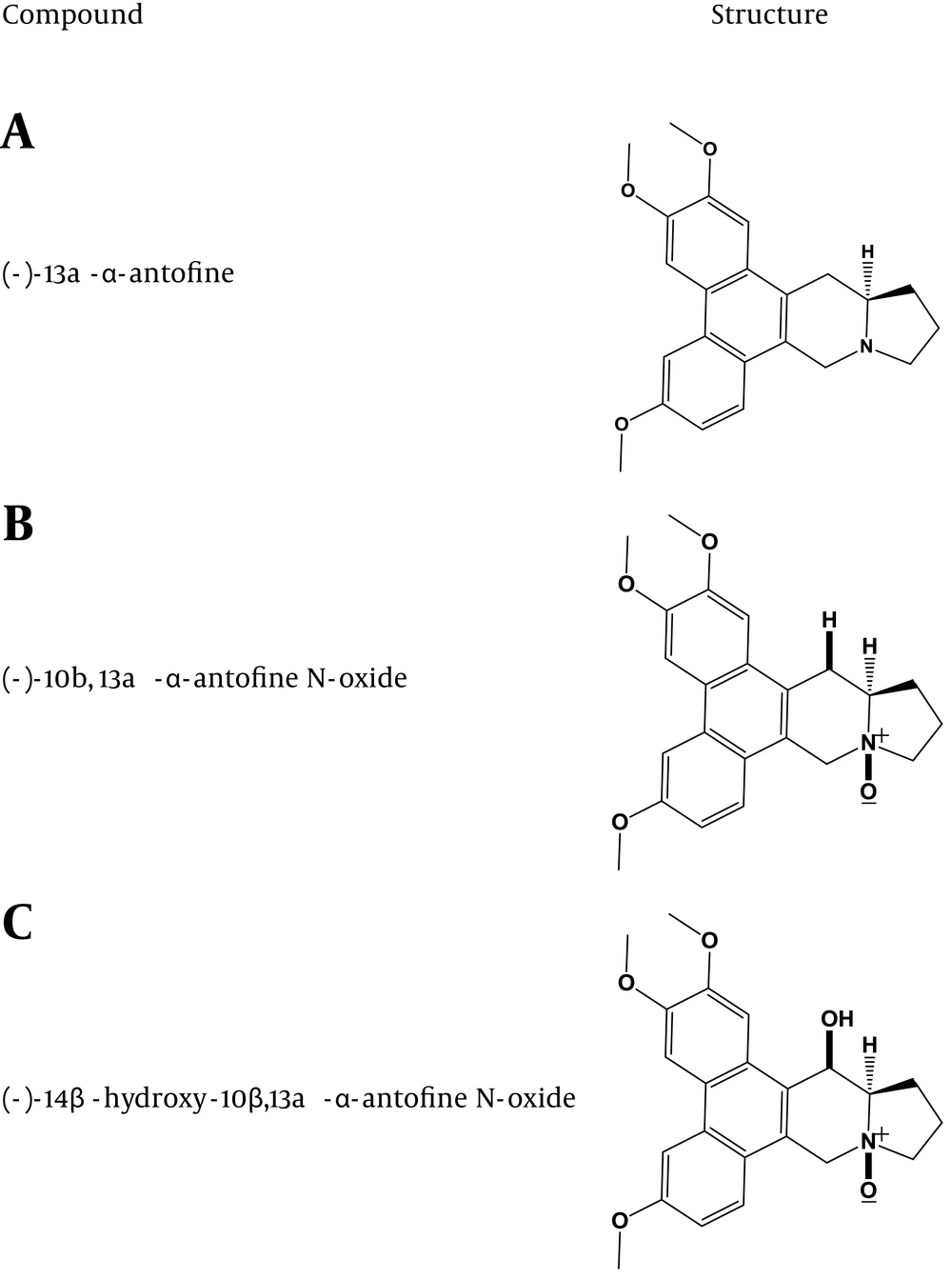
Vincetoxicum spp. contains numerous phenanthroindolizidine alkaloids (16, 17). Several phenanthroindolizidine alkaloids, including (-)-13a-α-anto-fine, (-)-10b, 13a-α-antofine N-oxide, and (-)-14β-hydroxy-10β, 13a-α-antofine N-oxides were previously identified as major components of V. pumilum (15). The structures of these compounds are presented in (Figure 2). To date, there are no other reports in the literature about isolation/structures of the active agents in V. pumilum. However, several active agents have been documented in other Vincetoxicum species (see 11) and would presumably also be present in the V. pumilum used here.
4.2. Inhibition of Cell Viability
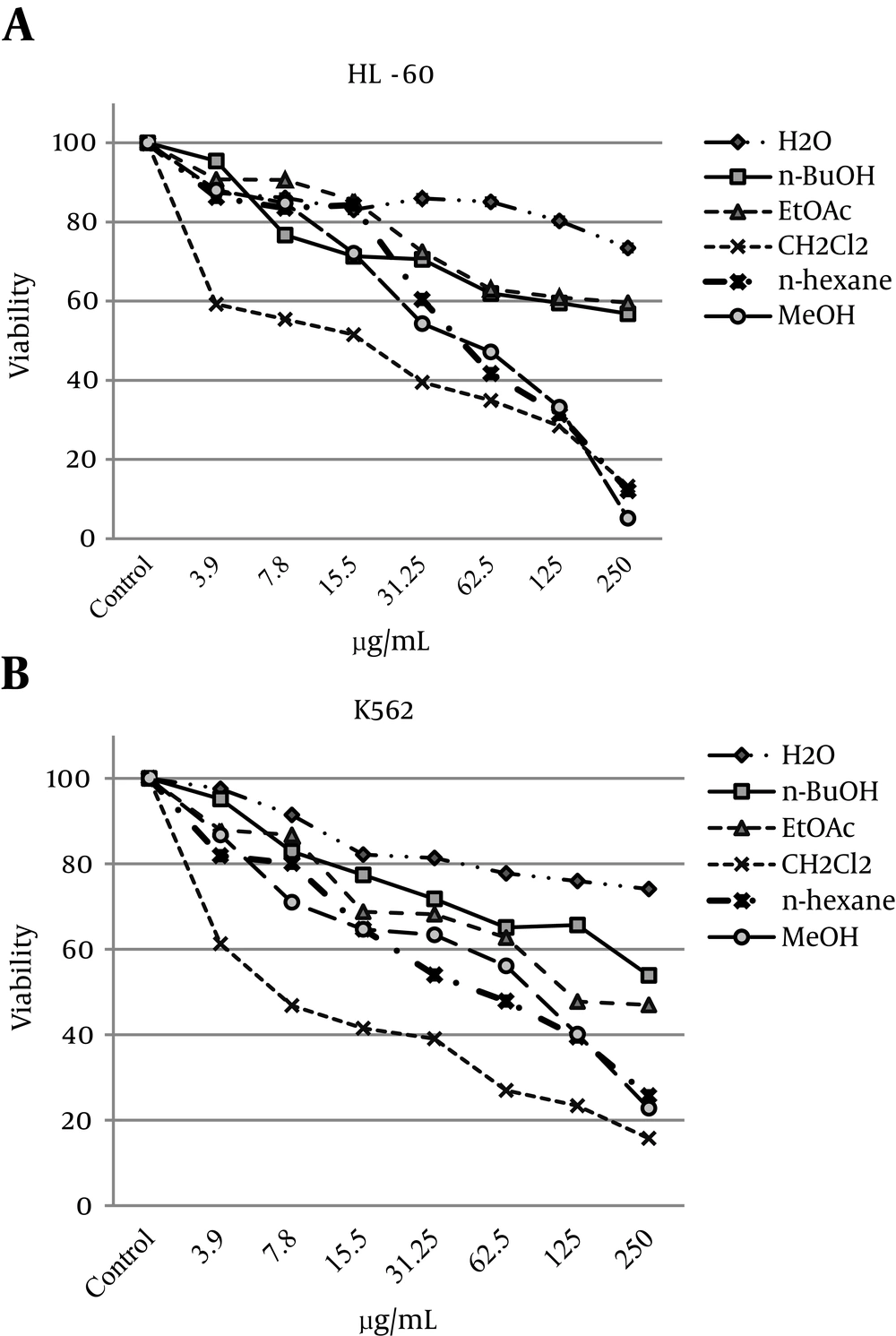
Inhibition of cell viability caused by the methanol extract of V. pumilum and its fractions was examined using an alamarBlue® assay. To compare the cytotoxicity of the methanol extract and its fractions, the HL-60 and K562 cells were incubated with different concentrations for 48 hours. The results showed that the methanol extract and both the CH2Cl2 and n-hexane fractions caused decreased viability of cells in a concentration-related manner (Figure 3A). The observed morphological changes included reductions in cell volume and rounding of the cells treated with the methanol extract or the CH2Cl2 or n-hexane fractions. The calculated doses inducing 50% cell growth inhibition (IC50) against the HL-60 and K562 cells for the methanol extract and each sub-fraction are presented in (Table 1). The cytotoxic effect of the methanol extract and the CH2Cl2 fraction against human lymphocytes isolated from peripheral blood was minimal (data not shown).
| Cell line | Fraction | IC50 | IC50 Range |
|---|---|---|---|
| HL-60 | MeOH | 43.8 | 34.3 - 55.9 |
| n-hexane | 49.2 | 40.5 - 59.7 | |
| CH2Cl2 | 12.5 | 7.3 - 21.7 | |
| EtOAc | > 250 | - | |
| n-BuOH | > 250 | - | |
| H2O | > 250 | - | |
| K562 | MeOH | 58.8 | 38.4 - 90.1 |
| n-hexane | 50.2 | 35.6 - 70.8 | |
| CH2Cl2 | 8.3 | 6.1 - 11.2 | |
| EtOAc | 147.2 | 90.0 - 240.8 | |
| n-BuOH | > 250 | - | |
| H2O | > 250 | - |
aCell viability was quantitated with the alamarBlue® assay.
4.3. Flow Cytometric Analyses of Apoptosis Induction by CH2Cl2 Fraction
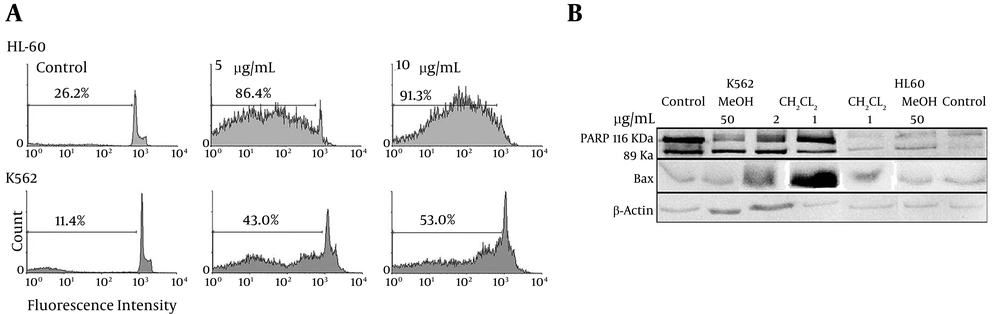
Apoptosis following treatment with the various extract/fractions was measured with PI staining and flow cytometry; the aim was to detect the presence of a sub-G1 peak resulting from DNA fragmentation. Both types of cells, when treated with 1.2, 2.5, 5.0, or 10.0 µg/L of CH2Cl2 fractions for 48 hours, displayed sub-G1 peaks in their flow histograms, an effect that was absent in the untreated cells (Figure 4A).
(A) Cells were treated with different concentrations of CH2Cl2 solvent fraction of V. pumilum (0.0, 5.0, and 10.0 µg/mL) for 48 hours, then subjected to staining and flow cytometric analyses. Sub-G1 peaks (indicative of apoptotic cells) were induced in treated cells but not in control cells. The treated cells exhibited a concentration-related effect, indicating involvement of the apoptotic process in fraction-induced cell death. (B) Proteolytic cleavage of poly(ADP-ribose) polymerase (PARP) and increasing Bax protein expression in HL-60 and K562 cells after 48 hours of exposure to the V. pumilum methanol extract (50 μg/mL) or CH2Cl2 fraction (1 and 2 μg/mL). β-actin was used as a loading control. Western blots are representative of three independent experiments.
4.4. Poly (ADP Ribose) Polymerase Cleavage and Expression of Bax Proteins
Cleavage of 116 kDa PARP-1 to 89 and 24 kDa fragments was used as an indicator of apoptosis in the treated cells. In K562 cells, PARP-1 was cleaved clearly to the two fragments after 48 hours of treatment with the methanol extract (50 μg/mL) or the CH2Cl2 fraction (1 and 2 μg/mL). Bax proteins, which play a pivotal role in controlling cytochrome c release and apoptosis initiation via mitochondrial pathways (21), were present in increasing levels only after treatment with the CH2Cl2 fraction (Figure 4B).
5. Discussion
Natural compounds derived from plants have served as invaluable resources for the prevention and treatment of a variety of human diseases, including cancer. Plant-based formulations have emerged as promising new sources for investigations of related molecular events. Detailed chemical analyses of certain botanical medicines have been performed to establish the safety and efficacy of these potential anti-cancer remedies (22). As several anti-cancer phytochemicals can alter the function of key molecules regulating programmed cell death, plants with apoptosis-modulating potential may ultimately prove useful as new chemotherapeutics (23). Considering the data on the in vitro cytotoxic activity of phenanthroindolizidine alkaloids from V. hirundinaria (16, 17) and the chemical similarity among plants in this genus, V. pumilum was chosen for investigation of potential cytotoxic and apoptotic properties. To our knowledge, there are no reports to date in the literature about these effects of V. pumilum. Nevertheless, the data from this study showed that a methanol extract of V. pumilum as well as some of its sub-fractions imparted potent inhibitory effects on cell viability in HL-60 and K562 human leukemia cell lines.
A cytotoxic evaluation of the entire extract could yield insights into the biological activity of any natural product, itself a complex mixture of variable components with different physicochemical properties. In our case, partitioning the V. pumilum total methanol extract into the various n-hexane, CH2Cl2, EtOAc, n-BuOH, and H2O sub-fractions separated the phytochemicals present, according to polarity (24). The fact that higher activity was seen with the parent V. pumilum methanol extract and in the n-hexane and CH2Cl2 fractions suggested that non/semipolar compounds imparted the cytotoxic effects in the test cell lines. Low-polarity solvents yield extracts that are often successfully absorbed through the cell membrane (15). As such, it would be expected that the low-polarity solvents effectively concentrated a majority of the cytotoxic compounds in the V. pumilum materials in this study. Accordingly, the n-hexane and CH2Cl2 fractions did have the greatest level of cytotoxic activity.
Chemicals and plant extracts that can induce apoptosis are considered potential anti-cancer agents (1). In this case, due to the findings with regard to potent cytotoxic activity in the various cell lines studied here, the n-hexane and CH2Cl2 fractions were further evaluated for apoptotic activity. Apoptosis occurs through multiple events in cells. Caspases interact with other molecules and regulate their activation; effector caspases are activated as a consequence of initiator caspase activation. A clustering of interactions automatically cleaves targets of caspases, including nuclear lamins, ICAD/DFF45, PARP, and PAK2. Cleavage and inactivation of these and other target molecules ultimately results in fragmentation of DNA, resulting in the ability to detect a characteristic ‘DNA ladder’ in these cells. Because apoptotic cells lose small fragments of DNA and take up less stain, in terms of flow cytometric analysis, these cells will appear to the left of the G1 peak when they are stained with a quantitative DNA-binding dye, such as PI (20). In the present study, the CH2Cl2 fraction of V. pumilum resulted in an increase in the overall percentage of sub-G1 peaks in flow histograms, indicating that there had been an amplification of apoptosis in these cells.
The balance between pro-apoptotic and anti-apoptotic proteins in the outer layer of mitochondrial membranes regulates cellular apoptosis. Bax is a pro-apoptotic molecule up-regulated by the tumor suppressor protein p53, and has been shown to be involved in p53-mediated apoptosis. Interacting with Bax, p53 promotes its activation and insertion into the mitochondrial membrane (17). Here, treatment with the methanol extract and the CH2Cl2 fraction of V. pumilum effectively increased Bax protein levels in both cell lines in comparison to the control cells. In addition, each of these preparations also resulted in greater cleavage of cellular PARP. PARP is normally inactivated when cleaved into the 24 and 89 kDa segments (the DNA-binding and auto-modification domains, respectively) by caspases (primarily caspases 3 and 7 in vivo). Because DNA binding can still occur by the smaller fragment, its ability to dissociate from DNA is lost without the presence of the larger counterpart; this prevents intact PARP from accessing damage sites in DNA and triggering repairs as needed. With enough accumulated damage, programmed cell death, including possibly apoptosis, could then occur.
As noted earlier, V. pumilum contains a myriad of constituents, including phenanthroindolizidine alkaloids, that have been postulated as imparting the cytotoxic properties seen in other members of the genus (15-17). As noted in Staerk et al. (2005) (16), “it has been shown that the toxicity of phenanthroindolizidine alkaloids is due to inhibition of protein and nucleic acid synthesis.” Among the various agents in the methanol extract and the n-hexane/CH2Cl2 fractions, it is likely that the alkaloids (-)-13a-α-antofine, (-)-10b,13a-α-antofine N-oxide, and (-)-14β-hydroxy-10β,13a-α-antofine N-oxide were the major components responsible for the effects observed in this study on cell viability in general and apoptosis induction in particular. This would be in keeping with results of earlier studies whose authors indicated that the presence of alkaloids such as antofine was important to impart potent anti-tumor cell activity (25, 26). Interestingly, recent evidence has indicated that some of these alkaloids are also useful as anti-inflammatory agents capable of blocking the release of inflammatory mediators by cultured macrophages via effects on cell-signaling pathways (27). Whether similar effects on signaling pathways are part of the mechanism(s) related to the V. pumilum induction of apoptosis and overall cytotoxicity remains to be determined.
5.1. Conclusions
This study established for the first time that the herb V. pumilum imparted an inhibitory effect on cell viability and that this effect was mediated, at least in part, by the induction of apoptosis in human leukemia cells. Considering the important role of apoptosis-inducing agents in novel therapeutic strategies against cancer, the results confirmed our hypothesis that this natural product could have a potential useful role in the treatment of at least some types of cancer. Clearly, future research is necessary to better characterize the constituents of the active fractions in the current study, and to permit a better understanding of the mechanisms by which this natural product works. With that knowledge, more precise studies could then be designed to test for and control the toxicities of various fractions or the whole herb of V. pumilum prior to any wider use in clinical settings, including anti-cancer treatments.