1. Background
In ancient times, before the development of new chemical antimicrobial agents, medicinal plants were used by indigenous people for treatment of their infectious diseases. Currently, the antimicrobial activities of plants for treatment of infections have been scientifically approved (1-5).
Satureja khuzestanica (Lamiaceae family) is extensively grown in northern Khuzestan and southern Lorestan provinces of Iran (6). The people from these regions know S. khuzestanica as an analgesic and antiseptic herb since ancient times (7).
The folklor and traditional healers have recommended taking a cup of Satureja infusion before the meals to treat many ailments such as muscle pains, indigestion, cramps, nausea, diarrhea, and infectious diseases, especially bladder infections and inflammations (7, 8). Recent studies have confirmed the anti-inflammatory (9), analgesic (10), and many traditional uses of this plant.
Escherichia coli has been reported as the cause of 62.5% of urinary tract infections (11). It can cause a variety of infections such as urinary tract infections (12, 13), neonatal meningitis (14, 15), nosocomial blood stream infections (16), genital tract infection of pregnant women (17), neonatal nosocomial infections (18), catheter associated infections in patients undergoing hemodialysis (19), bloodstream infections (20) and ophthalmia neonatorum (21) in both humans (14-16, 22-26) and animals. Furthermore, a high frequency of antibiotic resistant E. coli strains has been reported from different parts of the world (15, 27, 28).
In one study from northern west of Iran, the resistant E. coli strains were reported to amoxicillin (99.3%), gentamicin (33.6%), tetracycline (72.8%), ceftazidime (46.4%), co-trimoxazole (75%), imipenem (1.4%), ciprofloxacin (47.6%), norfloxacin (50.7%), cephalothin (77.8%), amikacin (12.1%), nitrofurantoin (12.9%), chloramphenicol (20.7%), and nalidixic acid (60.7%). It has been shown that 84.2% of these isolates were multi-drug resistant E. coli (27).
2. Objectives
Because of the importance of E. coli role in urinary tract infections and high frequency of drug resistant E. coli, we evaluate the antibacterial activity of Satureja khuzestanica essential oil against clinical isolates of E. coli extracted from the patients with urinary tract infections.
3. Materials and Methods
3.1. Plant Materials and Essential Oil Extraction
Full flowering Satureja khuzestanica aerial parts were collected from Poldokhtar, Lorestan province, Iran in June 2014. The plants were dried in shade. Then, the herbarium samples were identified and authenticated under number 168-1 in the agricultural department, research center of Barij, Kashan, Iran. The plant was powdered and subjected to hydrodistillation by Clevenger-type apparatus for 3 hours. The essential oil was separated and kept in a dark vial at a cold place until its analysis.
3.2. Analyses of the Essential Oil by GC and GC-MS Analyses
The chemical composition of essential oil was analyzed using gas chromatography (GC) and mass spectrometer (GC-MS) apparatuses. The GC and GC-MS analyses were conducted by Agilent technology (HP) 6890 with capillary column of HP-1MS (30 m × 0.25 mm, film thickness 0.25 μm) and Agilent technology (HP) 6890 coupled with 5973 network mass selective detector system, respectively. The oven temperature program was initiated at 40°C, held for 1 minute and then raised up to 230°C at a rate of 3°C/minute, held for 10 minutes. Helium was used as the carrier gas at a flow rate of 1.0 mL/minute with a split ratio equal to 1/50 injector. The detector and injector temperatures were 250°C and 230°C, respectively. Components of essential oil were identified through comparison with retention index (RI) relative to homologous series of n-alkanes and by computer search using libraries of Wiley 275.L and Wiley 7n.1, as well as comparing the fragmentation pattern of the mass spectra with data published in the literature (29).
3.3. Escherichia coli Strains and Antimicrobial Evaluations
In this study, we used 15 clinical isolates of Escherichia coli, including 14 clinical isolates of E. coli from patients with urinary tract infections and one ATCC 8739. They were identified by biochemical test and culturing on Eosin methylene blue (EMB) agar. E. coli strains were cultured overnight on nutrient Agar (Merck). One or two colonies of each strain were suspended in normal saline and its turbidity was adjusted to 0.5 MacFarland by spectrophotometrical method (1 × 108 CFU/mL).
The inhibition zone diameters (in mm) of S. khuzestanica essential oil were determined by disk diffusion assay. The above microbial suspensions were inoculated on Muller Hinton Agar (Merck) by sterile cotton swab. Sterile disks containing different concentrations of essential oil were placed on inoculated plates. The plates were incubated for 24 - 48 hours, then the inhibition zone diameters (mm) were measured and recorded as means ± standard deviation (SD) (30).
The minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) values of essential oil were determined by microbroth dilution assay. The essential oil was dissolved in DMSO (Dimethyl sulfoxide) and diluted in water and then 100 μL of each dilution was added into each well of 96-well microtiter plates. The above microbial suspensions were diluted to 106 CFU/mL. Afterwards, 100 µL of diluted microbial suspensions were added to each well and incubated at 37°C for 24 hours. The concentration of the first well not showing any turbidity was recorded as the MIC and the concentration of the first well without any growth on agar medium was reported as the MBC (31).
3.4. Checkerboard Titer Test for Gentamicin and S. khuzestanica Essential Oil Against E. coli
Eight serial, twofold dilutions of S. khuzestanica essential oil and gentamicin were prepared. Then, 50 µL of each dilution of essential oil or carvacrol was added to the wells of 96-well plates in vertical orientation and 10 µL of gentamicin dilutions was added in horizontal orientation.
One hundred µL of E. coli (106 CFU/mL) was added to each well and incubated at 35 ± 2°C for 24 hours. Fractional inhibitory concentrations (FICs) were calculated as the MIC of the essential oil and gentamicin combination divided by the MIC of essential oil or gentamicin alone. The FIC index was the total FIC of essential oil and gentamicin and interpreted as synergistic effect when < 0.5, indifferent when ≥ 0.5 - 2, and antagonistic when > 2.0. The synergic effect is shown graphically by applying published isobole methods (32).
3.5. Statistical Analyses
The data were analyzed in GraphPad Prism software and the differences between the means of inhibition zone diameters, MIC and MBC values were estimated by 1-way Analysis of variance (ANOVA). The significant levels between the means values were 0.05.
4. Results
4.1. Chemical Composition of S. khuzestanica Essential Oil
Eleven components were identified in S. khuzestanica essential oil that represented 98% of total essential oil composition. Carvacrol (94.1%) was the main component of S. khuzestanica essential oil followed by β-bisabolene (1.7%) and p-cymene (0.5%) (Table 1).
| Compound | RI | Percentage |
|---|---|---|
| α-Terpinene | 929 | 0.2 |
| p-Cymene | 938 | 0.5 |
| β-Phellandrene | 941 | 0.1 |
| γ-Terpinene | 971 | 0.4 |
| Linalool | 1013 | 0.1 |
| Carvacrol | 1257 | 94.1 |
| Eugenol | 1295 | 0.2 |
| trans-Caryophyllene | 1327 | 0.4 |
| α-Bergamotene | 1336 | 0.1 |
| Neryl-acetone | 1350 | 0.1 |
| β-Bisabolene | 1387 | 1.7 |
Major Components of Satureja khuzestanica Oil
4.2. The Antimicrobial Activity of S. khuzestanica Essential Oil Against Clinical Isolates of E. coli
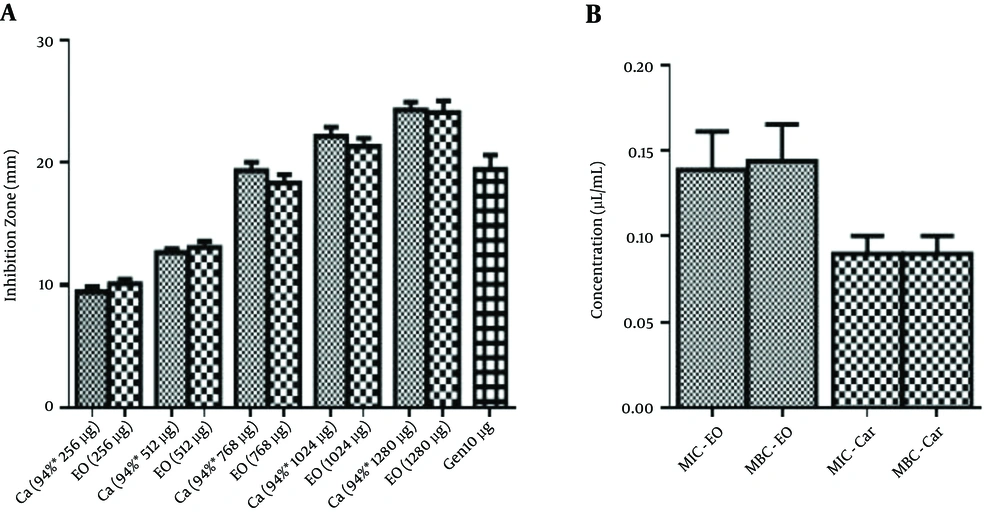
In this study, we evaluated the antimicrobial activity of different concentrations of S. khuzestanica essential oil and its main component, carvacrol against clinical isolates of E. coli by disk diffusion method (Figure 1).
In each concentration, the amount of carvacrol was equal to its concentration in S. khuzestanica essential oil. The inhibition zone diameters of carvacrol and S. khuzestanica essential oil were intensified with increasing their concentrations.
The inhibition zone diameters of carvacrol were not significantly different with the inhibition zone diameters of S. khuzestanica essential oil.
The inhibition zone diameters of S. khuzestanica essential oil (768 µg) and of carvacrol (721 µg, 94% of 768 µg essential oil) were equal to the inhibition zone diameters of gentamicin (10 µg). In higher concentrations of carvacrol or essential oil, the inhibition zone diameters of essential oil and carvacrol were significantly larger than that of gentamicin (Figure 1A).
The antimicrobial evaluation of essential oil and carvacrol by microbroth dilution assay showed that the mean of MIC values (µL/mL) for carvacrol and S. khuzestanica essential oil were 0.09 and 0.14, respectively while the MIC value for gentamicin was 2.3 µg/mL. The MBC values (µL/mL) for carvacrol and S. khuzestanica essential oil were 0.09 and 0.14, respectively, while the MBC value for gentamicin was 4.3 µg/mL. There was no significant statistically difference between the antibacterial activity of carvacrol and S. khuzestanica essential oil against clinical isolates of E. coli (Figure 1B).
4.3. The Synergistic Evaluation Between the Essential Oil and Gentamicin
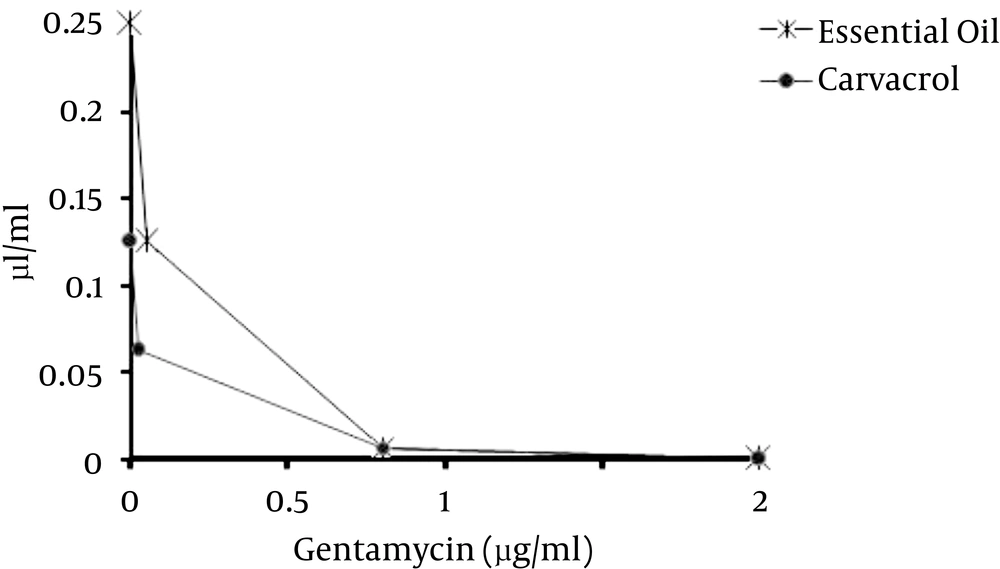
The synergistic effect of gentamicin with carvacrol was evaluated too. The presence of both carvacrol and S. khuzestanica essential oil decreased the MIC value of gentamicin to 0.0625. Gentamicin decreased the MIC value of carvacrol and S. khuzestanica essential oil from 0.25 and 0.125 to 0.007 and 0.007, respectively. The FIC of gentamicin with carvacrol and essential oil were the same and was 0.03125 while the FIC of carvacrol and S. khuzestanica essential oil were 0.028 and 0.0028, respectively (Table 2). FICI of gentamicin with carvacrol and S. khuzestanica essential oil were < 0.05. Therefore, carvacrol and S. khuzestanica essential oil had synergistic effects with gentamicin as isobologram curve is shown (Figure 2).
| FIC | FICI | |
|---|---|---|
| Gentamicin | 0.03125 | 0.05925 |
| Carvacrol | 0.028 | |
| Gentamicin | 0.03125 | 0.03405 |
| S. khuzestanica oil | 0.0028 |
The FIC and FICI Values of Gentamicin With S. khuzestanica Oil or Carvacrol Against E. coli ATCC 8739
5. Discussion
Carvacrol inhibits E. coli O157H7 (33) by different mechanisms such as down-regulating the genes expressed in colonization and invasion (34), inducing the heat shock protein 60, inhibiting the flagellin synthesis (22), disrupting depolarization of the cytoplasmic membrane, and increasing the permeability of membranes (35).
The results of our study showed that S. khuzestanica essential oil has high antibacterial activity against clinical isolates of E. coli, but the antibacterial activity of carvacrol against clinical isolates of E. coli was higher than S. khuzestanica essential oil. Presumably, the other minor components antagonize carvacrol effect in S. khuzestanica essential oil and decrease its antibacterial properties. Furthermore, S. khuzestanica essential oil had strong antibacterial activity against clinical isolates of E. coli and can be a suitable choice for treatment of urinary tract infections.
Today, the resistance of the clinical isolates of E. coli toward the current antibiotics (20, 36) has been increased. Thus, breaking this resistance by lowering the dose of antibiotics or minimizing the shots of treatments by essential oils provides a new horizon for effective treatments.
In this study, we confirmed the synergistic effects of gentamicin with carvacrol and S. khuzestanica essential oil. S. khuzestanica essential oil by its main component, carvacrol, decreased the MIC value of gentamicin to low dose. Therefore, further clinical investigations can be conducted to evaluate the synergistic effects of S. khuzestanica essential oil with gentamicin in the treatment of urinary tract infection.