1. Background
Among the patients with colorectal cancer, who underwent curative surgery, the cancer will eventually recur, in about half, leading to metastasis and death (1). Accordingly, treatment of colorectal cancer requires a multimodal approach, based on the stage of the disease (2). The benefit of adjuvant chemotherapy, based on 5-fluorouracil (5-FU) regimen, in limiting recurrence and increasing patient survival, is established (2). The FOLFOX regimen (5-FU, leucovorin, and oxaliplatin) is a current standard of care, for patients with stage III colon cancer (3). The 5-FU can be administered in the FOLFOX regimen, by either infusion or bolus protocols. In colorectal cancer patients, using infusion of 5-FU had equal or better response, while it has less toxicity, compared with bolus-based program (4-8). Moreover, the infusional approach has been associated with better quality of life and recovery time, in colorectal cancer patients (9). Accordingly, the current regimen applies a dual schedule, with bolus administration, followed by continuous-infusion of 5-FU, in order to achieve the maximum cytotoxic effects (6).
Previous studies have applied different duration of infusion, for administration of 5-FU within the FOLFOX regimen, ranging from 8 to 120 hours (10, 11). However, longer infusion duration requires prolongation of hospital stay, which can be overwhelming for patients, and limits their functions. Although infusion pumps are applicable for outpatient settings, such devices require a central venous line placement, which has its own complications (e.g. infection, blockage) (12). Long-term occupation of hospital beds, besides imposing costs on the healthcare system, also decreases resources because of the increasing need for chemotherapy (13). Therefore, if treatment efficacy is maintained and toxicity is not increased, reducing the time of 5-FU infusion will be valuable by decreasing the patient’s hospitalization time and associated costs.
2. Objectives
In this retrospective study, cancer treatment outcomes are compared between short-time infusion of 5-FU 8-hour infusion (5-FU 8h) and 22-hour 5-FU infusion (5-FU 22h), in colon cancer patients. We hypothesized that a shorter infusion time of 5-FU would be at least equal to the current protocol, in terms of treatment outcomes.
3. Patients and Methods
3.1. Patients and Settings
This retrospective study was conducted on colon cancer patients, who have been referred to two cancer treatment centers in Ahvaz city (Iran), between 2005 and 2011. Inclusion criteria for this study were as follows: 1) total colon resection for stage II/III tumor and a high risk that present at least one of the following situations: T4, intestinal perforation or obstruction, poorly differentiated tumor, venous invasion, or if less than 14 lymph nodes have been examined; 2) the most inferior part of the tumor being superior to the peritoneal reflection, 15 cm from the anal verge; 3) postoperative chemotherapy being started within 8 weeks after surgery; 4) no previous chemotherapy, immunotherapy, or radiotherapy; 5) acceptable renal and hepatic function and blood tests before chemotherapy; and 6) being treated with FOLFOX regimen including treatment for 2 days every 2 weeks, for at least 24 weeks. The study was approved by the ethics committee of the Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
3.2. Treatment Protocols
Patients in the two cancer centers of the study had received the same FOLFOX chemotherapy regimen (14), with different protocols in terms of 5-FU infusion duration. Accordingly, we were able to retrospectively compare the short-time infusion protocol (5-FU 8h) with the current infusion protocol (5-FU 22h). The first group (5-FU 22h) has been treated by the following protocol: treatment spread for 2 days, every 2 weeks, continued for 24 weeks. On the first day of the treatment, 2-hour infusion of oxaliplatin (85 mg/m2) was administered simultaneously with 2-hour infusion of leucovorin (200 mg/m2), using Y shaped infusion sets. This was followed by the bolus administration of 5-FU (400 mg/m2) and continued with infusion of 5-FU 22h (600 mg/m2). Administration of 5-FU/leucovorin was repeated in the second day. The second group (5-FU 8h) has been treated with the same chemotherapy regimen, with the infusion lasting for 8 hours, instead of 22 hours.
3.3. Measurements
Patient assessments, after chemotherapy, included measurement of carcinoembryonic antigen (CEA) every 3 months, for 3 years and, thereafter, every 6 months, in the 4th and 5th year. Thorax and abdomen computed tomography (CT) scans have been performed in the first 3 years and colonoscopy, at every 3 to 5 years. When reviewing the follow-up data, we considered secondary colon cancer as a recurrence, only if occurring at colorectal regions. Patients have been followed for up to 5 years, and, therefore, 3 and 5 year treatment outcomes could be provided. We considered the first endpoint as disease free survival (DFS), which was defined as the time between the end of chemotherapy and recurrence or death (whichever occurs first). The study’s secondary endpoint was considered as the overall survival (OS), defined as the time between the end of chemotherapy and death.
3.4. Statistical Analysis
Data were analyzed using the SPSS software for windows, version 16.0 (SPSS Inc., Chicago, IL, USA). Data are presented as mean ± standard deviation or number (%). Normal distribution of quantitative data was checked by the Kolmogorov–Smirnov test. Then, Independent Sample t-Test or Mann-Whitney U test were applied, for comparison of quantitative data. The Chi-square test or Fisher’s Exact test were conducted for comparison of qualitative data. Survival analysis was performed using the Kaplan-Meier plot. Also, Cox-regression analysis was performed to control possible confounders. Analyses were performed, based on per-protocol and intention-to-treat principles. A P < 0.05 was considered significant, in all analyses.
4. Results
A total of 144 patients were eligible to be entered into the study. Data of 36 patients were not complete to be included into the analyses. Finally, data of a total of 108 patients were analyzed, comprising of 58 patients in the 5-FU 8h group and 50 in the 5-FU 22h group. Comparisons of the two study groups, with regards to demographic data and cancer characteristics, are summarized in Table 1. There was no significant difference between the two groups, except for a higher number of evaluated lymph nodes (P = 0.008) and higher tumor grade, in the 5-FU 8h group (P = 0.039).
| Characteristics | 5-FU 8h, n = 58 | 5-FU 22h, n = 50 | P |
|---|---|---|---|
| Age, y | 51.3 ± 13.2 | 50.2 ± 12.6 | 0.671b |
| Gender | 0.569c | ||
| Male | 30 (51.7) | 23 (46.0) | |
| Female | 28 (48.3) | 27 (54.0) | |
| Cancer Site | 0.438c | ||
| Cecum | 15 (25.9) | 8 (20.0) | |
| Right colon | 10 (17.2) | 8 (20.0) | |
| Transverse colon | 3 (5.2) | 3 (7.5) | |
| Left colon | 11 (19.0) | 3 (7.5) | |
| Sigmoid | 19 (32.8) | 18 (45.0) | |
| Unknown | 0 | 10 | |
| No. of evaluated lymph nodes | 7.7 ± 5.4 | 4.5 ± 4.0 | 0.008d |
| No. of involved lymph nodes | 1.2 ± 1.5 | 1.0 ± 1.7 | 0.397d |
| LN ratio (involved/evaluated) | 0.16 ± 0.25 | 0.16 ± 0.24 | 0.968d |
| Tumor size, cm | 5.0 ± 1.9 | 5.5 ± 2.5 | 0.290b |
| Stage Group | 0.440c | ||
| II | 32 (55.1) | 23 (46) | |
| III | 26 (44.8) | 27 (54) | |
| Surgical Margin | 0.404c | ||
| Free | 56 (100) | 37 (97.3) | |
| Involved | 0 | 1 (2.6) | |
| Unknown | 2 | 12 | |
| Grade | 0.039c | ||
| Well differentiated | 13 (24.1) | 18 (50.0) | |
| Moderately differentiated | 35 (64.8) | 15 (41.7) | |
| Poor differentiated | 6 (11.1) | 3 (8.3) | |
| Unknown | 4 | 14 | |
| Lymph/Vascular Invasion | > 0.999c | ||
| Lymphatic invasion | 3 (7.5) | 2 (12.5) | |
| Vascular invasion | 14 (35) | 2 (12.5) | |
| Lymph-vascular invasion | 0 | 3 (18.7) | |
| No invasion | 23 (57.5) | 9 (50) | |
| Not mentioned/Unknown | 18 | 34 | |
| Perineural Invasion | 0.115c | ||
| Invasion | 1 (5) | 3 (27.2) | |
| No invasion | 19 (95) | 8 (61.5) | |
| Not mentioned/Unknown | 38 | 39 | |
| Obstruction | 0.348c | ||
| Yes | 5 (13.5) | 7 (23.3) | |
| No | 32 (86.4) | 23 (76.6) | |
| Not mentioned/Unknown | 21 | 20 |
aData are expressed as mean ± SD or No. (%).
bt-Test.
cChi-square/Fisher’s Exact Test.
dMann-Whitney U Test.
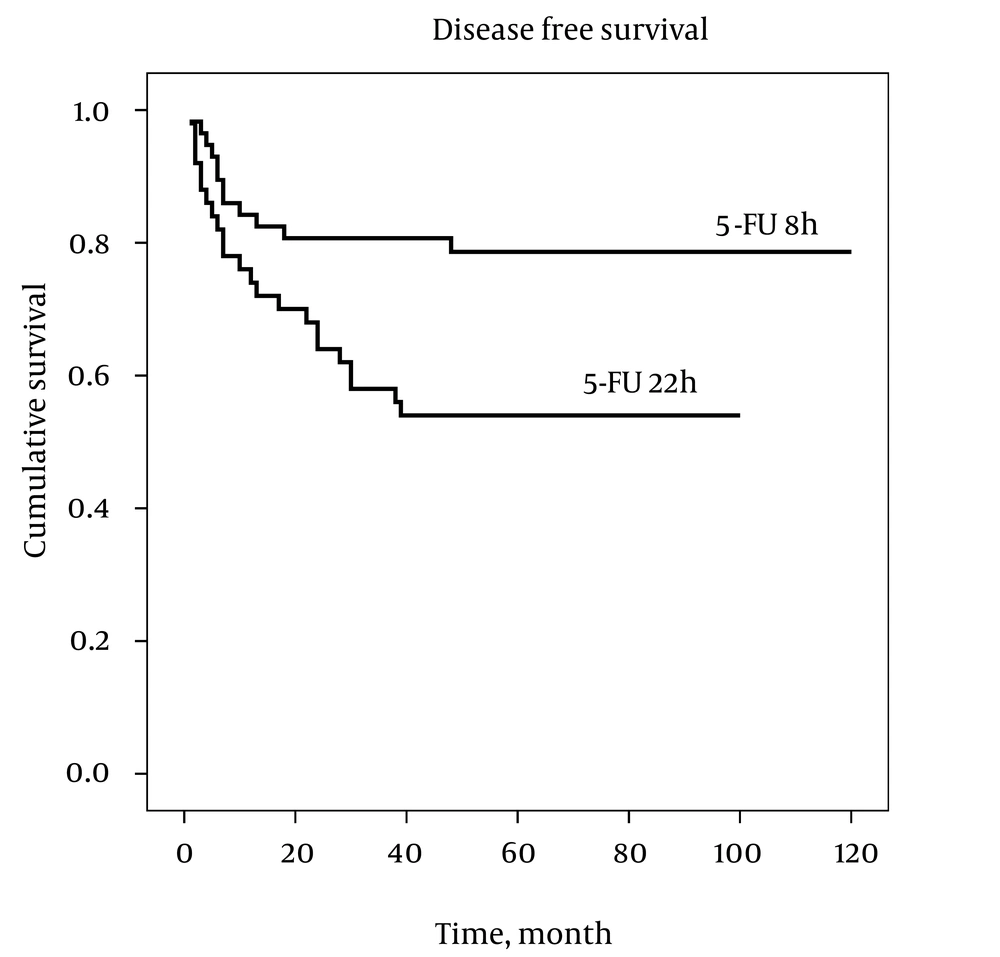
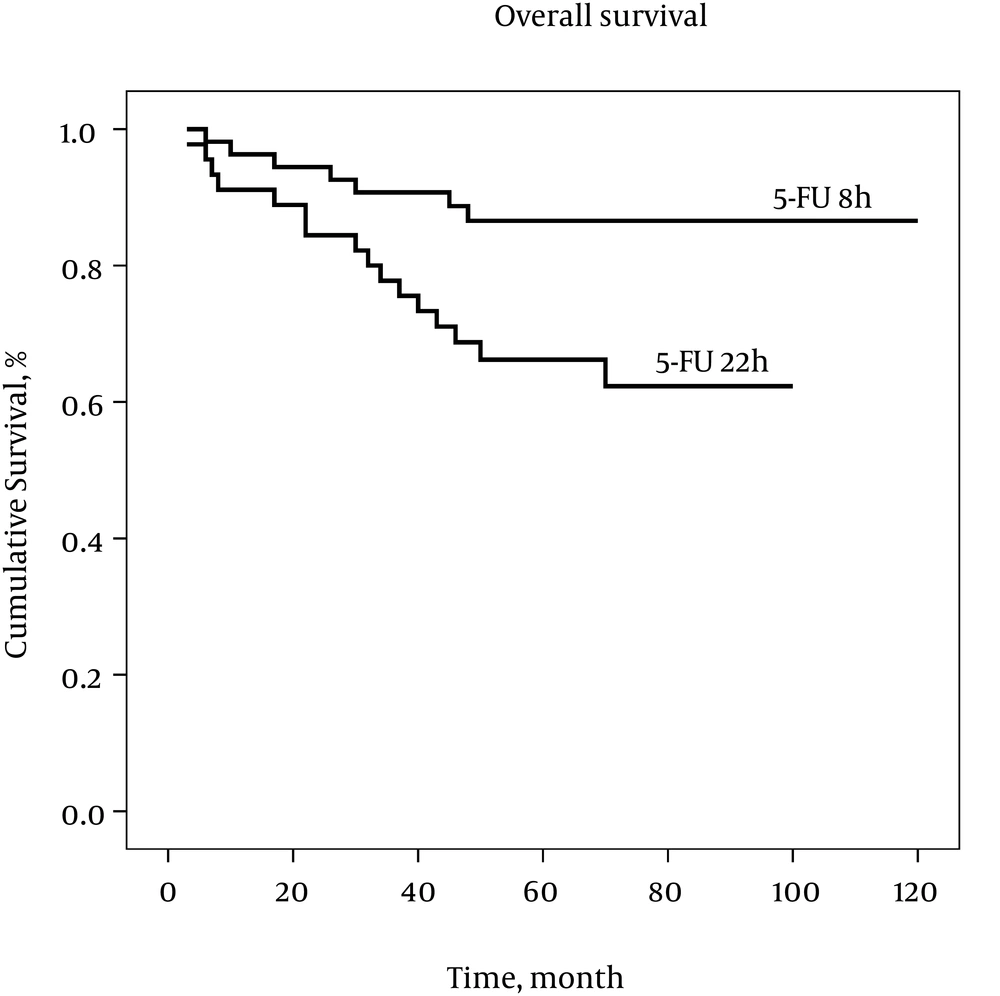
Comparisons of the study endpoints, based on the per-protocol principle, are summarized in Table 2. We were able to follow the patients for a mean duration of 4.7 ± 1.9 years (median = 5 years, ranged from 3 months to 10 years), with no difference between the two groups (P > 0.05). Relapse has occurred in 34 (31.4%) of the patients, which was lower in the 5-FU 8h group (18.9% vs. 46%, P = 0.004). Total mortality rate was also lower in the 5-FU 8h, compared with the 5-FU 22h group (14.5% vs. 36.9%, P = 0.011). Three and 5 years DFS was higher in the 5-FU 8h than 5-FU 22h group (P < 0.05), as depicted by Table 2. There was also a trend toward higher OS, in the 5-FU 8h group (P < 0.1), as seen in Table 2. Based on the intention-to-treat analysis, also, there was a lower overall mortality (44% vs. 22.4%, P = 0.023) and overall relapse (46% vs. 18.9%, P = 0.004), as well as a higher 3 years DFS (81% vs. 58%, P = 0.011) in the 5-FU 8h, compared with 5-FU 22h group, Table 3.
| Study Endpoints | 5-FU 8h, n = 58 | 5-FU 22h, n = 50 | P |
|---|---|---|---|
| Disease free survival evaluation time, month | 50.9 ± 25.7 | 45.1 ± 32.3 | 0.298b |
| Overall survival evaluation time, month | 57.2 ± 19.5 | 56.8 ± 27.0 | 0.937b |
| Overall mortality | 8 of 55 (14.5) | 17 of 45 (37.7) | 0.011c |
| Overall relapse | 11 (18.9) | 23 (46%) | 0.004c |
| Disease free survival, 3y | 47 of 56 (83.9) | 29 of 50 (58.0) | 0.011c |
| Disease free survival, 5y | 31 of 42 (73.8) | 23 of 46 (50.0) | 0.028c |
| Overall survival, 3y | 49 of 54 (90.7) | 35 of 45 (77.7) | 0.094c |
| Overall survival, 5y | 32 of 40 (80.0) | 26 of 42 (61.9) | 0.091c |
aData are expressed as mean ± SD or No. (%).
bt-Test.
cFisher’s Exact Test.
| Study Endpoints | 5-FU 8h, n = 58 | 5-FU 22, n = 50 | P |
|---|---|---|---|
| Overall mortality | 13 (22.4) | 22 (44) | 0.023b |
| Overall relapse | 11 (18.9) | 23 (46) | 0.004b |
| Disease free survival, 3y | 47 (81.0) | 29 (58) | 0.011b |
| Disease free survival, 5y | 31 (53.4) | 23 (46) | 0.562b |
| Overall survival, 3y | 49 (84.4) | 35 (70) | 0.103b |
| Overall survival, 5y | 32 (55.1) | 26 (52) | 0.846b |
aData are expressed as No. (%).
bFisher’s Exact Test.
Analyses for DFS and OS by the Kaplan–Meier estimator are presented in Figures 1 and 2, respectively. The Log Rank test showed a significant difference between the two groups for DFS, as well as OS, over the study follow-up period (P < 0.05). Considering a difference between the two groups in tumor grading, a Cox Regression analysis was performed, controlling for this factor. For DFS, 5-FU 8h versus 5-FU 22h was associated with better survival (Hazard ratio [95% CI] = 0.365 [0.160 to 0.833]), although no significant association was found between tumor grade and DFS (Hazard ratio [95% CI] = 1.339 [0.676 to 2.653]). With regard to OS, 5-FU 8h versus 5-FU 22h was associated with better survival (Hazard ratio [95% CI] = 0.286 [0.100 to 0.817]), while higher tumor grade was associated with worse survival (Hazard ratio [95% CI] = 2.638 [1.139 to 6.110]).
5. Discussion
Although 5-FU has been used as the cornerstone of chemotherapy regimens in gastrointestinal cancers, for several decades (2), the optimal administration schedule for this drug is still in debate. Because long infusion duration can significantly increase the healthcare costs, reducing infusion duration, while maintaining its efficacy, is of value. The aim of the present study was to investigate if infusion of 5-FU, over a shorter time (8 hours), is comparable to the current standard infusion protocols (22 hours) in the FOLFOX chemotherapy regimen. We found an overall lower relapse rate and lower total mortality rate, as well as better DFS, in the 5-FU 8h, compared with the 5-FU 22h group. These results indicate that a shorter duration of 5-FU infusion, in the FOLFOX chemotherapy regimen, may be applied instead of the current protocols, which could reduce hospitalization time and associated costs. However, better treatment outcomes, with the shorter infusion protocol, should be interpreted cautiously and need to be confirmed in prospective randomized clinical trials.
The administration schedule of 5-FU influences its cytotoxic effects. For example, while inhibition of DNA synthesis is more affected by the duration of exposure to 5-FU, RNA activities seem to be more influenced by the 5-FU peak concentration (15). Accordingly, two different schedules of 5-FU administration are applied concomitantly, to obtain optimal cytotoxic effects. Although studies have compared the bolus and continuous-infusion schedules of 5-FU, in terms of treatment outcomes and toxicity (4, 5, 9, 16), to our knowledge, there was no other report on direct comparison between various durations of 5-FU infusion, at the time of this study. We did not expect to observe better treatment outcomes with the shorter infusion protocol. The reason behind this finding is not clear. However, it may be related to the pharmacokinetic/pharmacodynamic properties of this drug (17).
The pharmacokinetic properties of 5-FU are influenced by the dose and schedule of drug administration. Clearance occurs rapidly, after bolus injection of the drug, with a primary half-life of 8 - 14 minutes. Because drug metabolism, by the dihydropyrimidine dehydrogenase, is saturable, clearance increases as the dose rate decrease, and is faster with infusion, compared to bolus injection (15). Likewise, clearance of a short-time infusion of 5-FU may be slower than a protracted infusion, with the same total dose, which leads to higher drug exposure during infusion and, eventually, more cytotoxic effects (15). This possible mechanism may partially explain our observation in this study that, at the same dose, a shorter infusion duration of 5-FU has better treatment outcomes, compared to a longer infusion of the drug.
The 5-FU has a narrow therapeutic index, with high levels resulting in severe side effects and low levels lacking a therapeutic effect (18). Pharmacodynamic differences in host drug sensitivity may have a circadian pattern (19, 20). Clearance of 5-FU by drug-metabolizing enzymes has time-dependent changes (21-23). Therefore, the therapeutic index of 5-FU may vary over a 24-hours period. Evidence exists on circadian changes in 5-FU plasma levels, by prolonged drug infusions (21, 24). A higher plasma concentration is observed in the evening, compared with lower levels, in the early morning (15), though studies have had controversial results, in this regard (25). This variability in 5-FU plasma concentration may affect variability in tolerance to the drug and lower therapeutic efficacy (15). Accordingly, several investigators have tried to alter the rate of a prolonged infusion in a circadian pattern, in order to improve the therapeutic index, called chronomodulation (26-28). However, previous studies have failed to find a constant circadian pattern of 5-FU plasma concentrations over prolonged drug infusion (17). In contrast to constant rate infusion of 5-FU, over a 22-hours period, in which there is substantial variability in 5-FU plasma concentrations, a 8-h period of infusion may be associated with less variations and better drug exposure. Accordingly, improved treatment outcomes, by the short-time 5-FU infusion, observed in our study may be explained by these pharmacokinetic/pharmacodynamic properties of 5-FU. This hypothesis should be investigated in further studies, by measuring and comparing the plasma concentrations of 5-FU, over two infusion schedules.
If our results with short-time 5-FU infusion in the FOLFOX regimen are confirmed by pharmacokinetic studies and prospective trials, it will have applications for chemotherapy of colon cancer patients. Future studies will discover if plasma concentration of 5-FU follows a circadian pattern (28). Therefore, short-time infusional 5-FU chemotherapy can be scheduled in a time interval, with low clearance leading to higher drug exposure and optimal cytotoxic effects. Also, synchronizing this infusional 5-FU chemotherapy around the time of the radiation therapy may enhance the toxic therapeutic ratio, in patients undergoing combined modality therapy (16).
Our study had a number of limitations. It was retrospectively conducted, with a limited sample size. The two investigated protocols have been conducted in two different centers, which might affect the results. Also, we had no reliable data on possible toxicities and side effects of the two protocols, which is one of the main concerns in modulating the 5-FU schedules.
In summary, we found an overall better treatment outcome in those colon cancer patients who had been treated with 5-FU infusion, over a shorter time (8 hours), compared with those who had received the protracted infusion program (22 hours), in the FOLFOX chemotherapy regimen. These observed effects may be attributed to the pharmacokinetic/pharmacodynamic properties of the drug. If superiority or non-inferiority are confirmed by pharmacokinetic studies, as well as prospective clinical trials, a shorter infusion of 5-FU, besides reducing healthcare costs, will also improve survival of the patients. Accordingly, studies are required in this regard.