1. Background
Many chemical carcinogens are able to generate free radicals by enzymatic reactions (1, 2). The relationship between cancer and free radicals has been studied (3, 4). Antioxidant compounds have been shown to slow down the development of chemically induced tumors (5-8). It has been estimated that the most of human cancer cases are due to environmental factors and would be preventable if the main risks could be identified (9, 10). The role of diet is important and a number of studies have suggested that absence of certain dietary compounds, notably antioxidants, contribute to increasing the risk of malignancy (11-14). There has been much interest in vitamin E, a biologically active phenol. Studies in animals have shown that vitamin E can prevent the growth of tumors induced in animals by chemical carcinogens (15-17). The potential of vitamin E as a cancer preventive agent has been studied and individuals whose intakes of vitamin E were above average showed a low risk of lung cancer (18). Vitamin E acts as a radical scavenger which inhibits peroxidation (19). It has been shown that in vitro, the oxidation of low density lipoprotein takes place only after complete consumption of vitamin E (20).
Biological membranes may be damaged by oxidation through chain reactions that generate lipid peroxyl radicals. Oxidation of the membrane results in impaired biological function and may lead to cell death (21-23). Vitamin E is the most important lipid-soluble biological antioxidant which scavenges peroxyl radicals and inhibits lipid peroxidation in vivo (24).
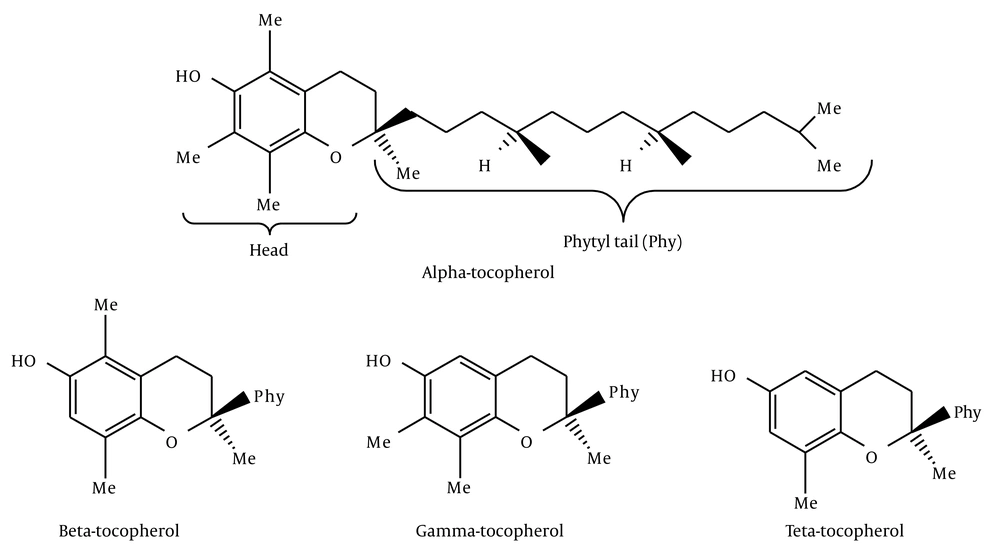
Vitamin E is a mixture of four lipid soluble phenols designated as α-, β-, γ-, and δ-tocopherol (Figure 1).
Tocopherols have terpenoid structure and they have a vitamin character because they do not originate in animal tissues. Vitamin E is a naturally occurring preservative found in vegetable oil, especially in wheat germ oil (25, 26). Vitamin E is a very important biological antioxidant and it is generally accepted that its most effective component is α-tocopherol.
2. Objectives
In our study, to investigate the stabilization of α-tocopherol-derived radicals in comparison to other phenoxyl radicals, a number of substituted phenols were synthesized, most of which contained t-butyl groups in 2- and 6-positions.
3. Materials and Methods
Chemical reagents were of analytical grade, purchased from Merck Co. (Germany) and all the synthesized compounds were recrystallized. The nuclear magnetic resonance (NMR) spectra were run on Bruker GRYOSPES WM 360 machine and the infrared (IR) spectra were run on a Perkin Elmer 1430 ratio recording infrared spectrophotometer. Microanalyses were carried out on a Perkin Elmer 4000 CHN analyzer; all the melting points were measured by an electrothermal melting point apparatus, and were uncorrected.
3.1. Preparation of Vitamin E Analogues
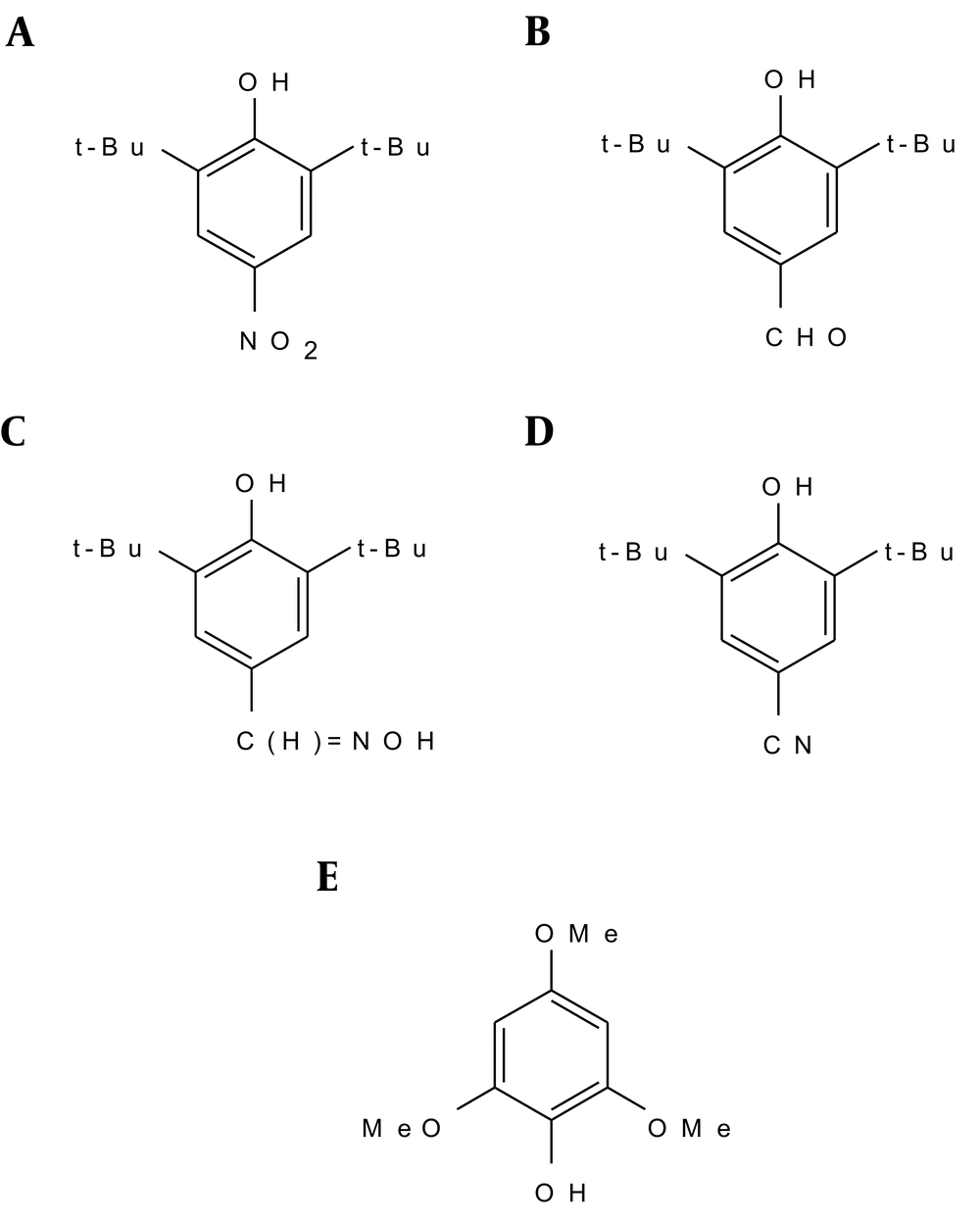
3.1.1. 2, 6-Di-t-Butyl-4-Nitrophenol
Fifteen milliliters of a 1:1 (v/v) mixture of nitric acid and acetic acid was added dropwise during 20 minutes to a stirred solution of 25 g of 2, 6-di-t-butylphenol in cyclohexane (75 mL). Barnes et al. (27) made the solution of 2, 6-di-t-butylphenol in acetic acid instead of cyclohexane. During the reaction, the solution was kept at 12 - 18°C. The solid 2, 6-di-t-butyl-4-nitrophenol was filtered, washed with water, and dried (Figure 2).
3.1.2. 2, 6-di-t-Butyl-4-Formylphenol
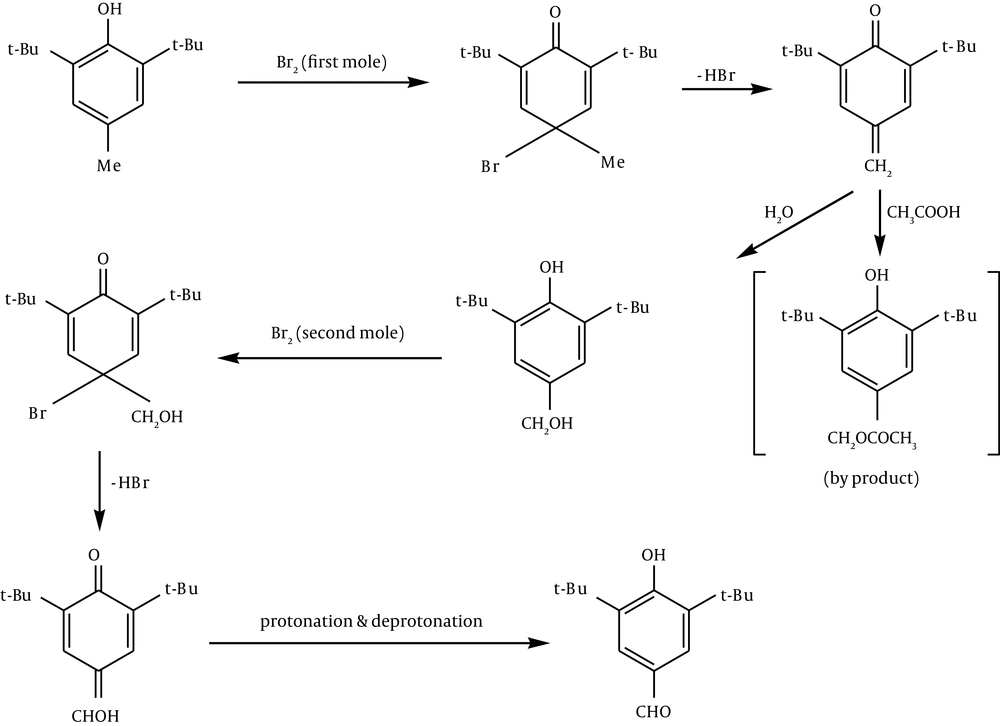
2, 6-di-t-butyl-4-methylphenol (80 g) was dissolved in glacial acetic acid (800 mL) and the solution was diluted with water (200 mL). T-butyl alcohol was used as the solvent instead of acetic acid by Coppinger et al. (28), in their preparation of 2, 6-di-t-butyl-4-formylphenol. Bromine (37 mL, 2 equivalents) was added dropwise with mechanical stirring over two hours. The suspension was diluted with ice water (500 mL). The mixture was kept at 0°C for several hours and was then filtered. The pale yellow product was washed with 50% acetic acid and water and was dried under reduced pressure (Figure 2).
3.1.3. 2, 6-di-t-Butyl-4 Aldoxime Phenol
The oxime was prepared from crude 2, 6-di-t-butyl-4-formylphenol by refluxing 2,6-di-t-butyl-4-formylphenol (30 g, 0.128 mol) with hydroxylamine hydrochloride (17.6 g, 0.253 mol), pyridine (40.3 g) and ethanol (75.6 mL) for three hours at 100°C, with stirring, while the reaction was in progress. After cooling, the product was shaken with H2O (150 mL), ether (20 mL), and NaCl (10 g). Muller et al. (29) prepared the oxime this way, but they did not separate it. The oxime-containing phase (upper layer) was evaporated down to give a colorless oil, which was dissolved in petroleum-ether [80 - 100°C] (130 mL), and concentrated by removing the solvent under reduced pressure until it became syrupy, but did not crystallize (Figure 2).
Recrystallization from petroleum-ether (60 - 80°C) gave a semi-solid material. This was dissolved in hot ethanol, and then diluted with water (dropwise) until the solution became cloudy. One further drop of ethanol was added, and after cooling, colorless crystals of the oxime separated and dried under vacuum at 98°C for 12 hours.
3.1.4. 2, 6-di-t-Butyl-4-Cyanophenol
A solution of 2, 6-di-t-butyl-4 aldoxime phenol (12.45 g, 0.05 mol) in 25 mL of acetic anhydride was heated on a steam bath for two hours. Then, the solution was poured into 1 M sodium bicarbonate (500 mL) and was stirred until the hydrolysis of the acetic anhydride was complete. The brown precipitate was extracted with 2 × 75 mL of methylene chloride, washed with sodium bicarbonate, and dried over sodium sulphate. Concentration by removing the solvent under reduced pressure afforded a brown solid material. The product was dissolved in ether and extracted with 1 M NaOH. The alkaline extract was acidified with 3 M hydrochloric acid and extracted with ether (Figure 2).
3.1.5. 2, 4, 6-Trimethoxyphenol
2, 4, 6-Trimethoxybenzaldehyde (0.981 g) and hydrogen peroxide (27.5%, 0.79 g) together with a catalytic amount of H2SO4 (98%, 0.1 mL) in methanol (10 mL) was stirred under argon atmosphere at room temperature for two hours. The solution was filtered and diluted with ether (30 mL). Extraction by 30 mL NaOH (2 M), followed by acidification of the aqueous layer with hydrochloric acid and extraction with 2 × 30 mL ether, gave a liquid product after evaporation of the solvent. This was dissolved in CH2Cl2 and purified by column chromatography (SiO2) (Figure 2).
4. Results
4.1. 2, 6-di-t-Butyl-4-Nitrophenol
The synthesized 2, 6-di-t-butyl-4-nitrophenol was then recrystallized three times from petroleum ether (40 - 60°C), giving 2, 6-di-t-butyl-4-nitrophenol as colorless needles (8.9 g, 29%), m.p. 154 - 155°C. δH (CDCl3) 1.46 (18 H, s, t-Bu), 5.91 (1 H, s, OH), and 8.10 (2 H, s, Harom). Found: C, 66.9; H, 8.4; N, 5.2. Calculated for C14H21NO3: C, 66.9; H, 8.4; N, 5.6%. The IR spectrum of 2, 6-di-t-butyl-4-nitrophenol in Nujol showed a sharp non-hydrogen bonded hydroxyl absorption at 3518 cm-1, and NO2 at 1595 cm-1.
4.2. 2, 6-di-t-Butyl-4-Formylphenol
Recrystallization of 2, 6-di-t-butyl-4-formylphenol from petroleum-ether (80 - 100°C), afforded pale yellow crystals, m.p. 189 - 190°C. δH (CDCl3) 1.46 (18 H, s, t-Bu), 5.82 (1 H, s, OH), 7.71 (2 H, s, Harom) and 9.83 (1 H, s, CHO). Found: C, 76.6; H, 9.5. Calculated for C15H22O2: C, 76.9; H, 9.5%. The IR spectrum of 2, 6-di-t-butyl-4-formylphenol in Nujol showed a hydroxyl absorption at 3400 cm-1, H-CO at 2715 cm-1 and C = O at 1652 cm-1.
4.3. 2, 6-di-t-Butyl -4 Aldoxime Phenol
The yield for 2, 6-di-t-butyl -4 aldoxime phenol was 14 g (44%), m.p.135 - 136°C. δH (CDCl3) 1.46 (18 H, t-Bu), 5.45 (1 H, s, OH), 7.27 (1 H, s, C = NOH), 7.40 (2 H, s, Harom), 8.08 (1 H, s, HC = NOH). The 1HNMR spectrum in D2O resulted in the exchange of protons by deuterium cations to give ArO-D and HC = NOD. The disappearance of the peaks at 5.45, 7.27 PPM indicated that these peaks were due to ArO-H and HCNO-H, respectively. The IR spectrum of 2, 6-di-t-butyl-4 aldoxime phenol in Nujol showed a hydroxyl absorption at 3300 cm-1 (phenol) and 3559 cm-1 (CH = NOH).
4.4. 2, 6-di-t-Butyl-4-Cyanophenol
The solvent was removed from the ether extract and the product was dried under vacuum and recrystallized from petroleum-ether (60 - 80°C), m.p. 144 - 145°C. δH (CDCl3) 1.44 (18 H, s, t-Bu), 5.75 (1 H, s, OH), and 7.47 (2 H, s, Harom). Found: C, 76.0; H, 9.0; N, 5.7. Calculated for C15H21NO: C, 77.9; H, 9.2, N, 6.1%. The IR spectrum of 2, 6-di-t-butyl-4-cyanophenol in Nujol showed a hydroxyl absorption at 3490 cm-1 and nitrile absorption at 2222 cm-1.
4.5. 2, 4, 6-Trimethoxyphenol
The phenol was obtained from the second and third fractions and after evaporation of the solvent the product was recrystallized from petroleum-ether as yellow needles, m.p. 64 - 66°C, δH (CDCl3) 3.75 (3 H, s, p-OCH3), 3.85 (6 H, s, o-OCH3), 5.08 (1 H, s, OH) and 6.17 (2 H, s, Harom). Found: C, 58.5; H, 6.5. Calculated for C9H12O4: C, 58.7; H, 6.6%. The IR spectrum for 2, 4, 6-trimethoxyphenol in Nujol shoed a hydroxyl absorption at 3434 cm-1, but because the methoxy peak was covered by the Nujol peak, another IR sample of 2, 4, 6-trimethoxyphenol in KBr was run, which showed hydroxyl absorption at 3434 cm-1 and methoxy absorption at 2844 cm-1.
5. Discussion
2, 6-Di-t-butyl-4-nitrophenol was obtained by electrophilic substitution involving N+O2.
In preparation of 2, 6-di-t-butyl-4-formyl phenol, it can be suggested that electrophilic addition of Br+ followed by loss of a proton leads to quinine.
Hydration of the methylene quinone gives out the phenol. Electrophilic addition of the second Br+ leads to methanol quinone followed by protonation, and deprotonation of quinone gives the formyl phenol (Figure 3).
In preparation of 2, 6-di-t-butyl-4-aldoximephenol, hydroxylamine condenses with the aldehyde group to give the oxime.
Dehydration of the oxime leads to formation of cyanophenol.
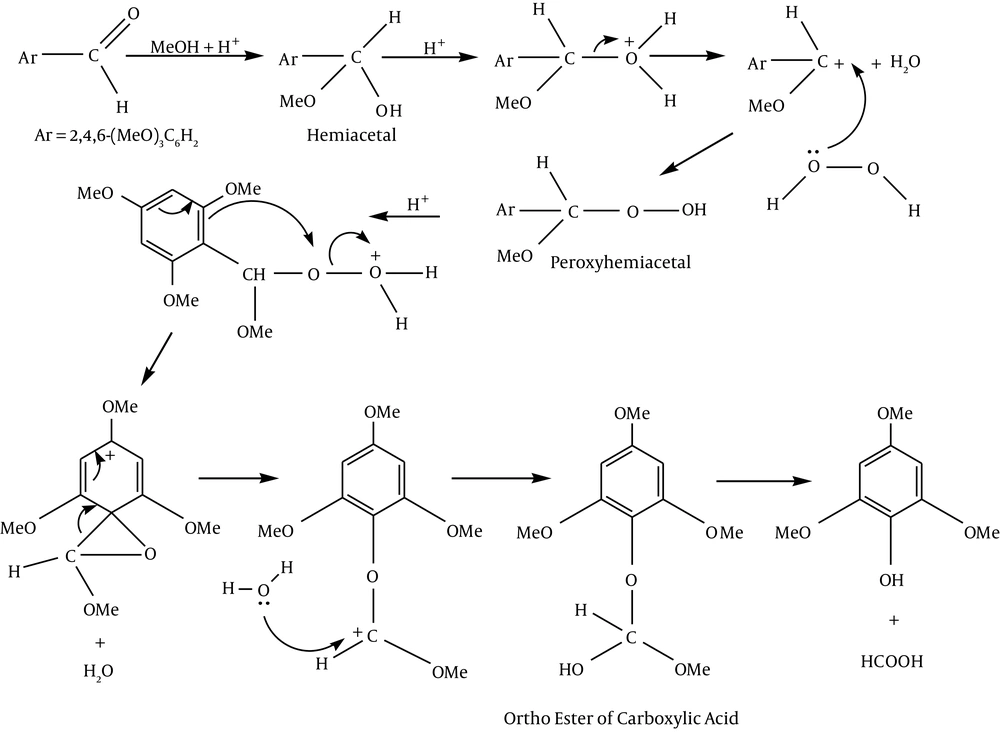
It can be suggested for the preparation of 2, 4, 6-tri-methoxy phenol that electrophilic addition of methanol to the aldehyde leads to hemiacetal.
Nucleophilic addition of hydrogen peroxide gives peroxyhemiacetal. Then protonation of peroxyhemiacetal followed by simultaneous dehydration and aryl migration gives the cation (aryl migration occurs to avoid the formation of an electron-deficient oxygen atom). After rearrangement, hydration leads to the ortho ester of the carboxylic acid. Loss of formic acid from the ortho ester provides phenol (Figure 4).
Autoxidation can cause damage to lipids and proteins. Antioxidant compounds have been shown to slow down the development of chemically induced tumors (30). There has been much interest in vitamin E as a radical scavenger which inhibits peroxidation. Vitamin E is a biologically active phenol which can easily convert to free radical and act as radical scavenger. Vitamin E radical is a phenoxyl radical which is more stable in comparison with an alkoxyl radical. This stability is due to the delocalization of the unpaired electron to the ortho and para position of the benzene ring.
The main point of our study was finding a compound much more reactive than phenol or vitamin E in terms of radical scavenger. For this purpose, we synthesized five vitamin E analogues to compare their efficiencies with vitamin E as an antioxidant to be able to interfere in the free radical chain reaction and terminate the propagation of free radicals. The efficacy of all five synthesized vitamin E analogues in terms of antioxidant activity and their kinetic study will be investigated in our other study.