1. Background
Celecoxib is a cyclooxygenase type 2 (COX2) inhibitor, which belongs to the non-steroidal anti-inflammatory drugs (NSAIDs). This drug has different pharmacological effects such as analgesic, anti-pyretic and anti-inflammatory (1-3). A previous study indicated that celecoxib exhibited central, not peripheral analgesia after systemic administration and its effect seemed to be mediated by opioid system rather than prostaglandin generation inhibition (4). Furthermore, it has been shown that other mechanisms (5, 6) including endogenous cannabinoids have contributed to the antinociceptive effect of celecoxib in intracerebroventricular (ICV) administration (6-8).
The endocannabinoid system has a key role in pain perception. Activation of CB1 and CB2 receptors in peripheral, spinal and supraspinal sites is responsible for antinociceptive effects of cannabinoids (9, 10).
It is revealed that endocannabinoids are metabolized by COX-2 and fatty-acid amide hydrolase (FAAH); so, probably, inhibition of COX-2 and FAAH by celecoxib could lead to raised endogenous cannabinoids level (11-13). However, other studies showed that endogenous cannabinoids contributed to antinociceptive effects of celecoxib in intracerebroventricular administration (6-8).
2. Objectives
Since the role of spinal cannabinoid receptors in antinociceptive effect of celecoxib is not clear, the aim of this study was to evaluate the possible involvement of spinal cannabinoid receptor in the antinociceptive effect of celecoxib in formalin test by the use of intrathecal administration of rimonabant, the CB1 selective receptor antagonist.
3. Materials and Methods
3.1. Animals
Male Wistar rats (140 - 180 g) were purchased from the central animal house of Jundishapur University of Medical Sciences (Ahvaz, Iran). The animals were maintained under 12-hours light/dark cycles in a temperature (20°C - 22°C) and humidity-controlled room with food and water provided ad libitum. Rats were acclimatized to the laboratory for at least one hour before testing and were used only once during the experiments. All the tests were carried out according to the institutional animal care and we used committee and ethical guidelines for investigations of experimental pain in conscious animals (14).
3.2. Drugs and Chemicals
Celecoxib (Razak Pharmaceutical Co., Iran), rimonabant (Sanofi Recherche, France) formaldehyde (Merck Co., Germany), and dimethyl sulfoxide (DMSO) (Sigma-Aldrich Co., St. Louis, MO, USA) were purchased. Celecoxib was dissolved in DMSO and administrated intrathecally (IT) in a volume of 10 μL/rat. Rimonabant was dissolved in DMSO and injected in a volume of 10 μL/rat IT.
3.2. Intrathecal Injections
IT catheterization was performed as described by Yaksh and Rudy (15). Animals were anesthetized with a mixture of ketamine hydrochloride (50 mg/kg) and xylazine (5 mg/kg) intraperitoneally. After creating the incision over the cervical spine, a polyethylene catheter (PE-10 (OD 0.55 mm, ID 0.30 mm) was inserted through an opening in the cisterna magna into the lumbar subarachnoid space (7.5 cm), and then the rift was closed. Rats with neurological disturbances were removed from the experimental procedures. IT drugs injection was performed by the use of microinjection syringe (OD 0.5 mm) over a 10-second period in a single injection in a volume of 10 μL followed by a flush of 10 μL normal saline (NS) in awake rats. For control group, vehicle was administrated as same as the treatment groups.
3.3. Formalin Test
The formalin test was carried out for the assessment of antinociception (16). Briefly, formalin (2.5% v/v) was administered by intraplantar (ipl) injection into the rat right hind paw in a volume of 50 μL. The rats were returned to the experimental cage and nociceptive behaviors were assessed. Pain score was given, described by Dubuisson and Denni: 0 = normal weight bearing on the injected paw; 1 = limping during locomotion or resting the paw lightly on the floor; 2 = elevation of the injected paw so that at most the nail touches the floor; and 3 = licking, biting or grooming the injected paw. The first five minutes of post-formalin injection was recorded as the early phase (neurogenic) and the period between 15 - 60 minutes was recorded as the late phase (inflammatory). Pain behavior was estimated through the area under the curve in time-pain score graph in 15-second intervals by trapezoidal rule (17). Lastly, the animals were euthanized.
3.4. Experimental Design
The first series of experiments was conducted to evaluate the antinociceptive activity of celecoxib in rat formalin test. Animals received celecoxib IT at doses of 0.5, 5 and 10 μg/rat. Then, the possible effects of rimonabant (100 and 200 µg) were determined. At last, the effects of rimonabant on antinociceptive effective dose of celecoxib were examined. To evaluate the antinociceptive effect of celecoxib on formalin-induced pain, rimonabant was administrated in doses of 100 and 200 μg/rat 10 minutes before celecoxib or vehicle injection. Ten minutes after celecoxib, the formalin test was performed.
3.5. Statistical Analysis
The data were presented as the means ± SEM and analyzed with Graphpad Prism 6 software. To analyze the differences, one-way analysis of variance (ANOVA) was used with post hoc Tukey’s test at P < 0.05.
4. Results
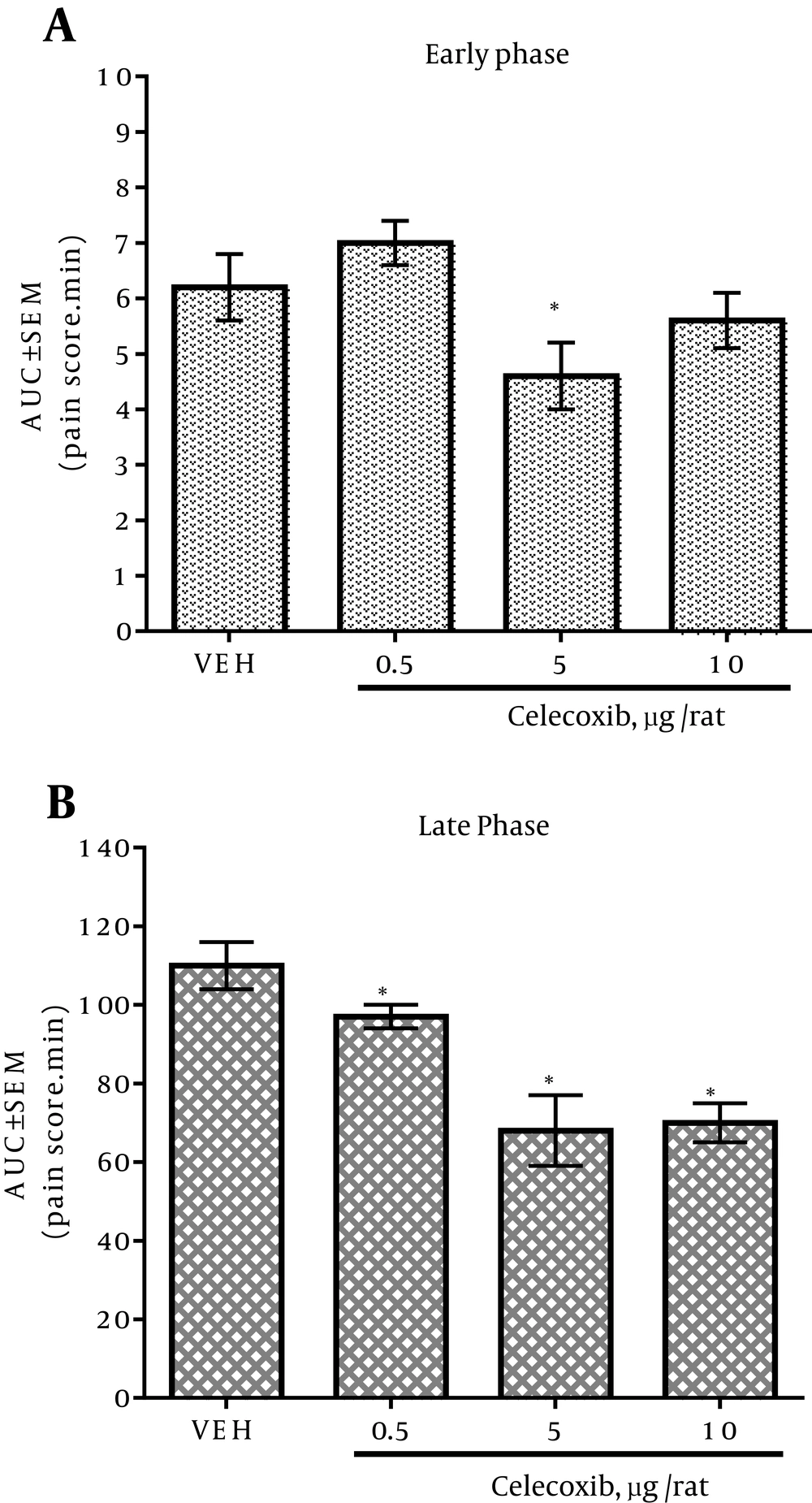
4.1. Effect of Intrathecal Celecoxib Administration on Formalin Test
In the vehicle-treated group, subplantar formalin administration into the right hind paw made a common pattern of nociceptive behavior, described as a biphasic time course. The early phase of the nociception started after formalin injection and lasted for five minutes. Fifteen minutes after the formalin injection, the late phase initiated and continued about 45 minutes. The IT injection of celecoxib caused attenuation in the pain score dose-dependently during the late phase of formalin test (Figure 1B). IT administration of 5 μg/rat of celecoxib decreased the pain score significantly in the early phase as compared with the vehicle group (Figure 1A). The IT injection of 0.5, 5 and 10 μg/rat of celecoxib decreased the pain score significantly in the late phase as compared with the vehicle group (Figure 1B). There was no significant difference in the antinociceptive activity between 5 and 10 μg/rat doses of celecoxib in the late phase of formalin test (Figure 1B).
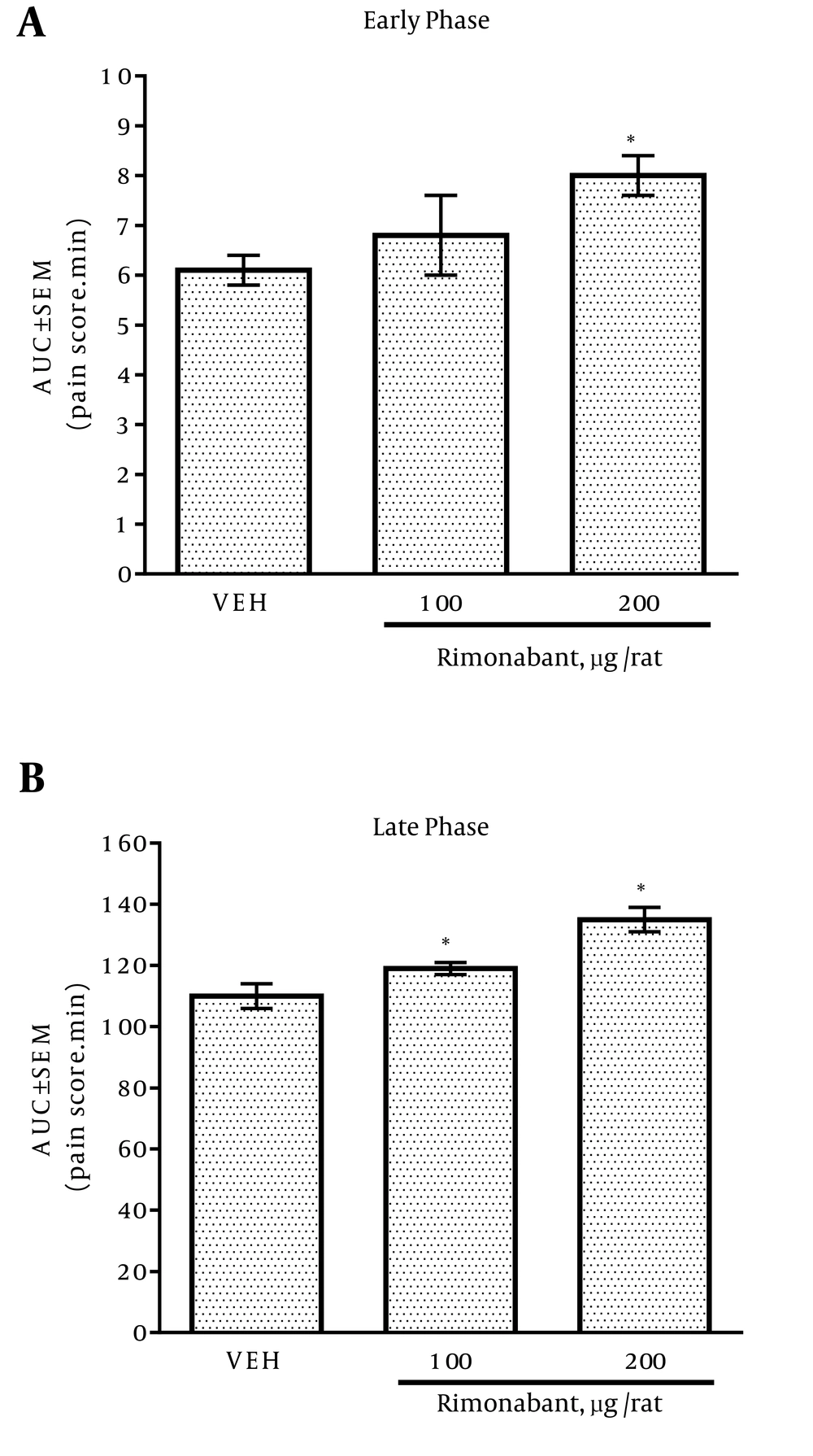
4.2. Effect of Intrathecal Rimonabant Administration on Formalin Test
IT administration of 200 μg/rat of rimonabant increased the pain score significantly in the early phase compared with the vehicle group (Figure 2A), whereas IT injection of 100 and 200 μg/rat of rimonabant significantly raised the pain score in the late phase compared with the vehicle group (Figure 2B).
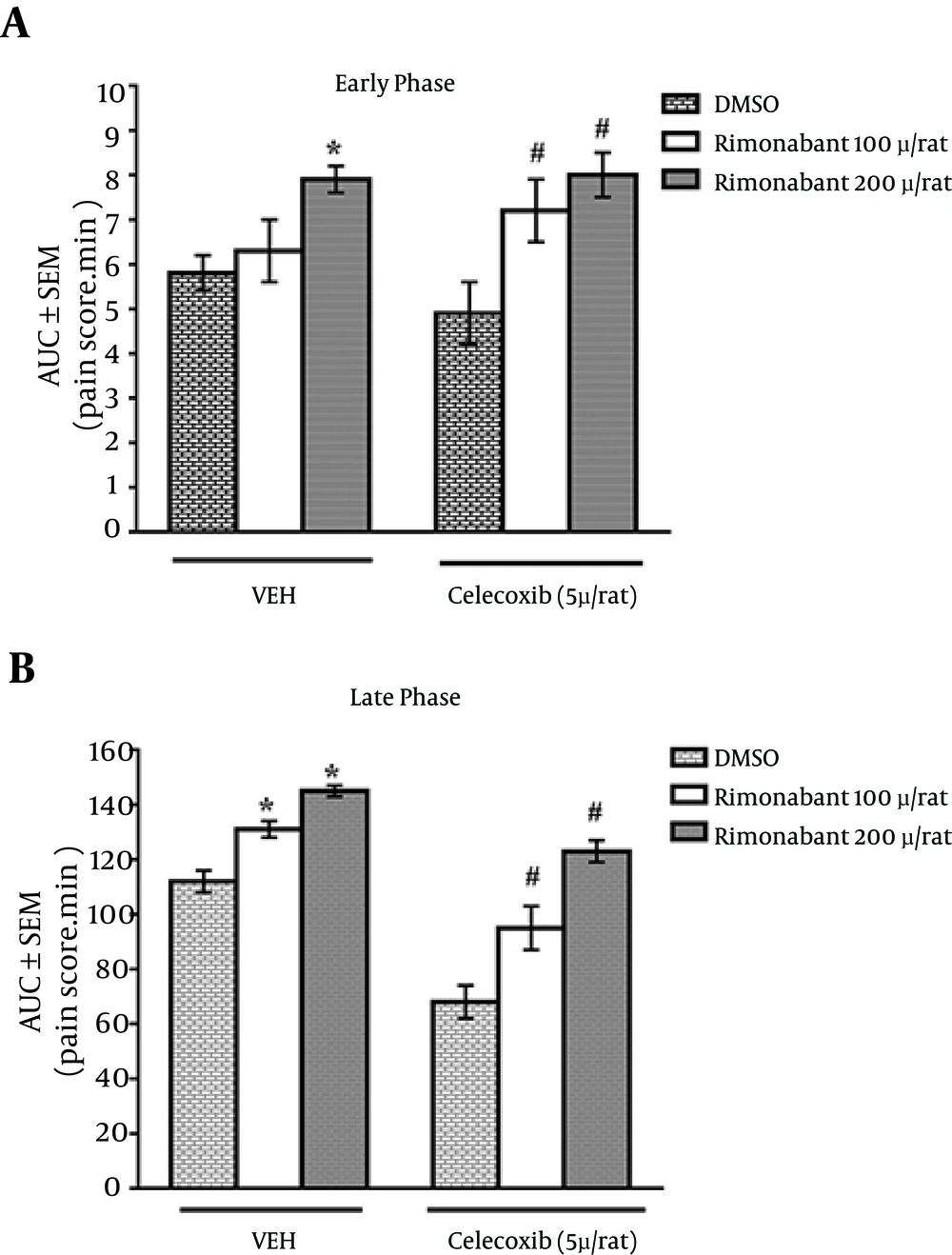
4.3. Effect of Rimonabant on the Antinociceptive Effect of Celecoxib
To evaluate the involvement of cannabinoid receptor in celecoxib antinociceptive effect, rimonabant (100, 200 μg/rat) was administrated IT 10 minutes before either celecoxib (5 μg/rat) or vehicle injection. As shown in Figure 3A and B, rimonabant could significantly reverse the analgesic effects of celecoxib (5 μg/rat) in both phases of the formalin test.
Data is expressed as the area under the curve of score-time curve in both phases of formalin test. Bars are the mean ± SEM of the data obtained in eight animals. Rimonabant or DMSO was administrated 10 minutes before celecoxib or vehicle. *P < 0.05 compared with the vehicle control group. #P < 0.05 compared with the non-treated celecoxib group.
5. Discussion
In this study, the role of spinal cannabinoid receptors in antinociceptive effect of IT administered celecoxib was evaluated, using formalin test. The constant nociceptive afferent barrage can lead to a progressive production of arachidonic acid in the dorsal horn available for conversion to mediators (18). The formalin test can lead to the increase of the arachidonic acid metabolites, which can affect the nociception responses (19). It was shown that anandamide could entirely inhibit the carrageenan-induced heat hyperalgesia (20).
The antinociceptive effects of NSAIDs are mediated through inhibiting the COX enzymes. Furthermore, other antinociceptive mechanisms such as interactions with endocannabinoids metabolism, leading to higher concentration of anandamide through shift of arachidonic acid metabolism to endocannabinoid production are involved (21-24). In addition, it was shown that NSAIDs could inhibit the enzymatic hydrolysis of anandamide by FAAH (21, 25); so, increased anandamide level may result in the reduction of pain behavior (13).
IT celecoxib (5 μg/rat) administration decreased the score of pain in early and late phases of formalin test. However, the IT injection of celecoxib (10 μg/rat) had no effect on the score of pain in early phase of formalin test. The study indicated that IT celecoxib had antinociceptive activity in late phase of formalin test in a dose-dependent manner. These data are in agreement with other studies in which IT celecoxib administration could inhibit nociception in both phases of formalin test (26-30).
Cannabinoid CB1 receptors are the major cannabinoid isoform receptors recognized in the central nervous system. Cannabinoid agonists and endocannabinoids exhibited strong antinociceptive activity via activation of CB1 in spinal cord level in different animal models of pain (31-33).
IT administration of rimonabant (100 and 200 µg/rat) resulted in hyperalgesia in late phase of formalin test; but in the early phase, only 200 µg/rat of rimonabant could induce hyperalgesia. This effect may be either a result of intrinsic inverse agonist of rimonabant in blocking the action of CB1 receptor or the inhibitory effect of rimonabant on antinociceptive function of endocannabinoids which was evoked by formalin test (34-37). However, it was reported that rimonabant could not produce hyperalgesia in rats (38).
Pretreatment with rimonabant (200 µg/rat) could reverse the antinociceptive effect of IT celecoxib (5 µg/rat) in both phases of the formalin test compared to the celecoxib group. These results revealed that the main antinociceptive effect of celecoxib was mediated by the cannabinoid system at the spinal cord level.
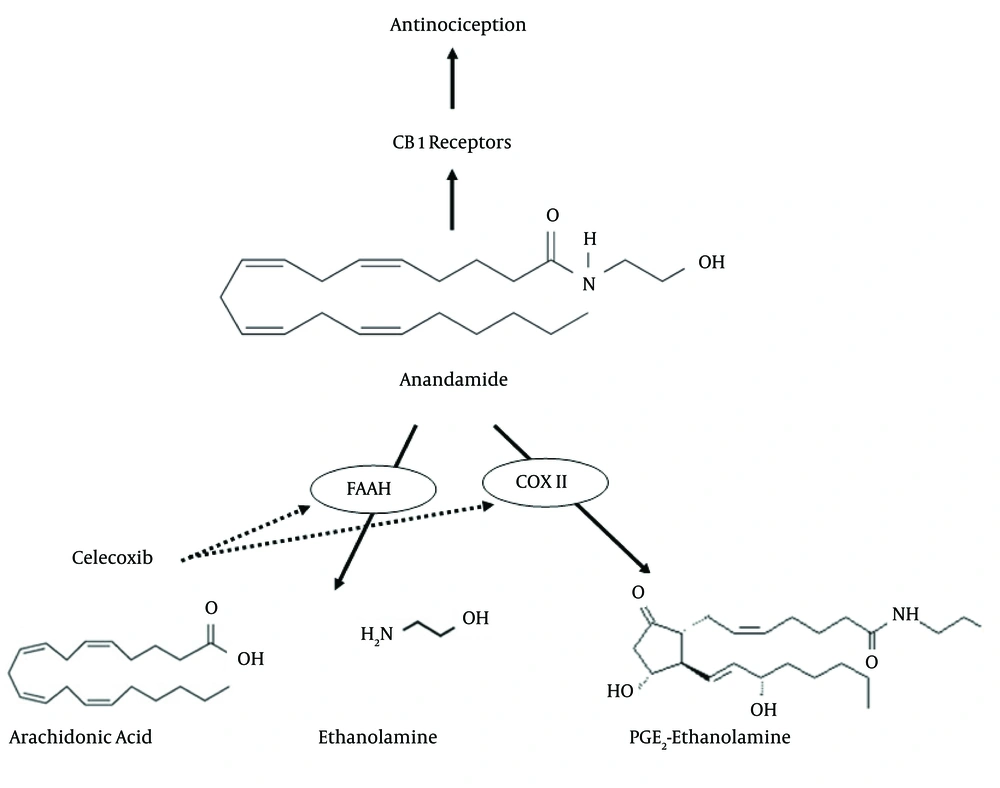
A probable interaction between the antinociceptive effect of COX inhibitors and endocannabinoids was investigated in previous studies. IT pretreatment with AM-251, the selective cannabinoid CB1 receptor antagonist, blocked the analgesic effects of indomethacin and flurbiprofen in animal pain models (22). The inhibition of COX-2 at the spinal cord level decreased both prostaglandin generation and endocannabinoid degradation and indicated that the endocannabinergic mechanisms was more responsible for their antinociceptive activities than the inhibition of spinal prostaglandin synthesis (39). Overall, the possible reaction pathways of celecoxib in this study are shown in Figure 4.
Generally, celecoxib inhibits COX II, the enzyme responsible for the conversion of arachidonic acid to prostaglandins and subsequently induction of the inflammatory pain. Furthermore, in the scheme, celecoxib prevents the metabolism of anandamide via inhibition of fatty acid amide hydrolase (FAAH) and COX II. These inhibitions increase the anandamide levels and subsequently cause more activation of CB1 cannabinoid receptors and more antinociception.
In conclusion, the present study suggested that endocannabinoids exhibited a key role in celecoxib antinociception at the spinal level in formalin test. Consistent with this, the antinociceptive effects of celecoxib was antagonized by CB1 receptor antagonist. Accordingly, the maintenance of the endocannabinoid level by COX II inhibition can potentiate the analgesic effects of celecoxib or other NSAIDs and highlight the importance combination use of cannabinoid agents and NSAIDs to increase their safety and efficacy.