1. Background
Microsporum, Trichophyton and Epidermatophyton are fungi that caused dermatophytosis. Dermatophytes are the pathogenic members of the keratinophilic soil fungi. Microsporum and Trichophyton are human and animal pathogens, while Epidermophyton is a human pathogen. They have the ability to attack keratinized tissues and used keratin and cause dermatophytosis (1, 2). Resulting diseases of these fungi often called Tinea. According to the World health organization (WHO), dermatophytes affect about 25% population of the world (3, 4). Although many antifungal drugs have been developed in recent years and have become available for the treatment of dermatophytosis, they are limited in two anti-fungal families: the allyl-amin and the azole. Because of their resistance and side effects in clinical, they can lead to failure in the treatment of mycoses (5, 6). The azole antifungal agents such as itraconazole or ketoconazole can be substrates and inhibitors of the P-glycoprotein, which excretes toxins and drugs into the intestines (7). Polyene antimycotics administration is limited by infusion-related toxicity, an effect postulated to result from proinflammatory cytokine production. The acute toxicity of amphotericin-B deoxycholate includes fever, rigors, nausea, hypertension, vomiting and hypoxia, and chronic side effect is nephrotoxicity (8). Synthetic fungicides can cause pollute in environment by their slow biodegradation in the environment. These years have seen a more interest to study about natural products, because they can be more efficacious than other already drugs in use and for diseases control and less harmful in environmental pollution. Probiotics, which are normal resident flora of intestinal tract, can be formulated into different types of products. The term “probiotic” was first proposed as microbial derived factors promoting the growth of other microorganisms in 1965 by Lilly and Stillwell (9). Probiotics have many useful effects such as lactose intolerance, prevention of atopic dermatitis, gastrointestinal disorders and urogenital infection. Probiotics have two mechanisms for their beneficial effects: 1) their live cells are caused direct effects, and 2) their metabolites; biogenics or metabolites are caused indirect effects. Lactic acid bacteria (LAB) are widely used in food and feed fermentation, contributing to the flavor, structure, safety and stability of food or feed products (10). Lactic acid bacteria are commonly used as probiotics (11, 12), which have potential for production of metabolites including enzymes, vitamins, organic acids, bacteriocins, and other metabolites (13). Several of these low molecular metabolites have been isolated and shown to have the ability to blunt or inhibit fungal growth (14, 15). Topical drug delivery is a useful painless route of drug administration, it offers an easy way for patients, and prevents the hepatic first pass metabolism of the drug (16, 17). Within the major groups of a topical drug delivery system, the use of topical gel formulation has expanded in pharmaceutical field because of its favorable properties such as being thixotropic and greaseless, easily spreadable and removed, emollient and water soluble (18). Hydroxy propyl methyl cellulose (HPMC) is recommended in the gel formulation because of its nontoxic nature, ease of compression and good bioadhesiveness (19).
2. Objectives
The purpose of this study was to investigate the effect of probiotic cell free extract on the dermatophytes and formulate a topical gel with effective concentration of probiotic extract as antidermatophyte preparations.
3. Materials and Methods
3.1. Microorganisms
The probiotic strain was lactobacillus casei (PTCC 1608). The freeze dried sealed glass ampoules of microorganisms used in this study were obtained from Persian type culture collection (PTCC). The microorganisms were as follows: Microsporum canis PTCC 5069, Microsporum gypseum PTCC 5070, Tricophyton rubrum PTCC 5143, and Tricophyton verocosum PTCC 5056.
3.2. Microbial Culture and Preparation of Fungal Inoculums
Bacteria were cultured in the MRS broth medium for 24 hours at 37°C and maintained on MRS agar, anaerobic glove box with 95% N2, 5% H2, 6% O2, and 5% CO2.These fungi were revived on saborad dextrose broth (SDB) and then cultured on saborad dextrose agar (SDA) at 22°C for 21 days at 22°C to obtain adequate growth.
3.3. Growth Rate Determination of Probiotics on Culture Media
For estimation of the growth rate of Lactobacillus casei, 1 mL (equal to102 cfu/mL) of the fresh cultured Lactobacillus was inoculated in 100 mL of the MRS broth. After mixing, flask was incubated at 37°C for 24 hours and viable cells count was performed.
3.4. Preparation of Cell-Free Supernatant
The Lactobacillus strain was grown anaerobically in 100 mL of the MRS broth for 24 hours at 37°C.Supernatant was obtained by centrifuging the medium at 3000 rpm for 15 minutes. Centrifuged supernatant was passed through a sterile 0.22 μ-pore-size filter unit (Millex GS Millipore). The filtrate was collected as the mixture of metabolites and then was kept at 4°C (20).
3.5. Lactic Acid Assay
Quantitative detection of lactic acid in acquired supernatant was performed using an enzymatic lactate reagent kit (chem. Enzyme). This assay method was based on two stage oxidative reactions as follows:


The absorbance of quinoneimine as the final colored product was measured at 500 nm wavelength (UV detector, S-3100, Scinco, Korea). The lactic acid level of supernatant was expressed as mg/dL. A standard curve was prepared using lactic acid with 99% of purity as the reference standard in different concentrations. The assay test was repeated in triplicate (20).
3.6. Long Term Stability Study
The acquired supernatant was stored for 9 months at 4°C. The value of pH was determined using a digital PH-meter (SL.901) during storage course and results were recorded.
3.7. Antidermatophytosis Effect of Supernatant
The agar well-plate method was used to determine an antidermatophytosis effect of acquired supernatant and its lyophilized form. This method needed fungal suspension with these characteristics; in λmax 580 nm with spectrophotometer (UV detector, S-3100, Scinco, Korea) the absorbance must be between 0.15 and 0.17 nm. Two bores were made on the pathogenic seeded medium in each petri-dish, and respectively, poured by 100 μL of fresh supernatant on 0 day (Sup-0) and the lyophilized probiotic extract (LPE). After keeping at 4°C for diffusion, the plates were incubated for 7 days at 22°C and a zone of inhibition was observed and measured (20).
3.8. Determination of the Minimum Inhibitory Concentration of Both Probiotic Extracts Against Dermatophytes Strains
The conventional macro serial dilution method was used to determine the minimum inhibitory concentration (MIC) of both probiotic extracts, fresh supernatant (Sup-0) and LPE with respect to four fungal strains. A stock solution of Sup-0 was prepared in sterile SDB (100 mg/mL), which was further diluted in SD-b to reach a concentration range of 6.25 mg/mL to 100 mg/mL. The lyophilized probiotic extract was also dissolved in SD-b to reach a concentration range of 0.0625 mg/mL to 1 mg/mL.
Final concentration of fungi in individual tubes was adjusted to about in 5 × 106 CFU/mL control tubes contained culture media with fungal strain (in 5 × 106 CFU/mL) and only culture media without any antifungal agent. Other test tubes included culture media with fungal strain (5 × 106 CFU/mL) and LPE, culture media with fungal strain (5 × 106 CFU/mL) and Sup-0.
After 7 - 10 days incubation at 22°C - 25°C, the test tubes were examined for possible fungal turbidity and MIC of both compounds for each test fungi was determined respectively as lowest concentration that could stop visible bacterial growth for 7 and 10 days (21).
3.9. Preparation of Hydroxy Propyl Methyl Cellulose Topical Gel
First, of total water needed was heated to 60°C, then HPMC powder was slowly added to preheated water with constant stirring. Then remaining water and precalculated amounts of LPE were added. The mixture was stirred until a homogenous appearance was attained. Then, each solution was left over night in the refrigerator until a transparent gel was formed (22).
3.10. Physical Examination
After the gels were set in the container, the homogeneity of each gel was tested by visual inspection. They were also examined for their physical appearance and the presence of any aggregation.
3.11. Determination of pH
Two gram of each gel formulation was dissolved in 10 mL of deionized water and their pH was measured using a digital PH-meter (SL.901). Each test was performed in triplicate and the results were reported as mean ± SE.
3.12. Viscosity
A Brookfield viscometer (model DV-II, spindle 64, 20 rpm) was utilized to measure the viscosity of gel formulations and evaluate their rheological behavior (23).
3.13. Grittiness
Each formulation was microscopically evaluated if any appreciable particle was seen under a light microscope (24).
3.14. Thermal Cycle Test
The portion were stored at 5°C for 48 hours and then at 25°C for 48 hours. The procedure was repeated 6 times and then their stability and appearance were evaluated (25).
3.15. Thermal Change Test
Three 20 g portion of each formulation were stored at 4 - 6°C, 25°C and 45 - 50°C. After 24 hours, one and three months, their stability and appearance were evaluated (25).
3.16. Freezing and Thawing
Twenty gram portion of each formula were stored periodically at 45°C - 50°C and 4°C for 48 hours each. The procedure was repeated six times and then the samples were checked regarding their appearance and stability (25).
Comparison of Antidermatophytophytic Effect of Gel Formulations With Clotrimazole Cream
The agar well plate method as describe previously, was used in this evaluation. Gels and cream must be diluted separately with distilled water (2: 3D.W). Then, equivalent amount of gels and cream (200 μL) were poured in wells separately. After 7 - 10 days incubation at 22°C, the zone of inhibition for each fungus was analyzed separately.
4. Results
4.1. Growth Kinetic of Lactobacillus casei
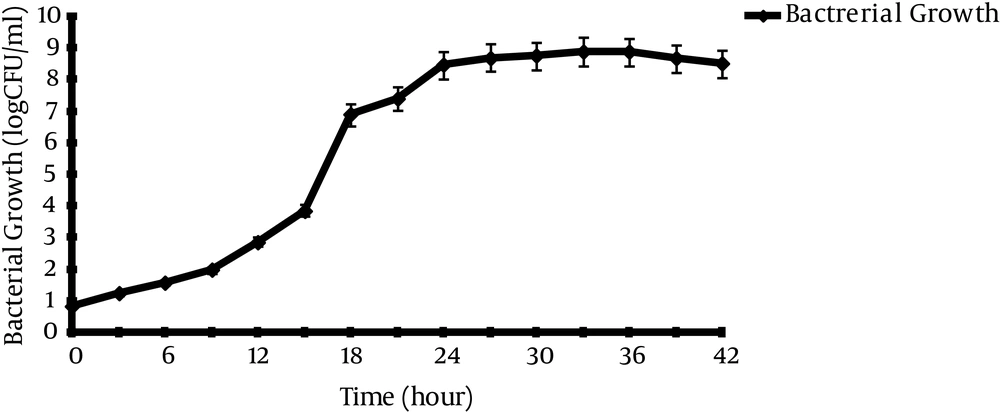
Figure 1 shows that Lactobacillus casei took approximately 8 hours to reach the log phase with generation time (TG) in 1 hour.
4.2. Long-Term Stability of Supernatant
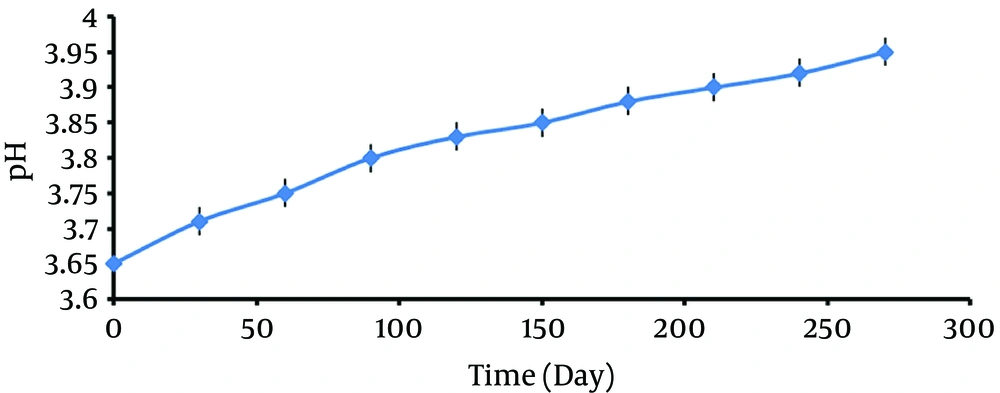
Evaluation of pH of the acquired cell free supernatant showed a few variations during 9 months storage in 4°C, but even these low changes could bias the stability of supernatant properties (Figure 2).
4.3. Antidermatophytosis Effect of the Supernatant
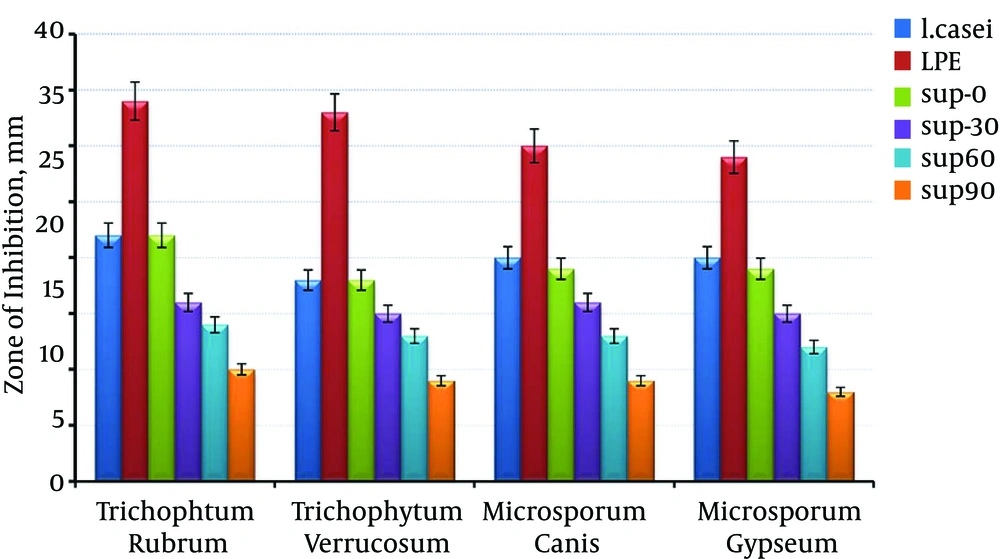
Figure 3 shows that, the antifungal activity of LPE was significantly higher than that of other groups, fresh supernatant (Sup-0) with probiotics viable cells and there was a significant reduction in effectiveness of antifungal properties of supernatant by time lapse as it was lowest in Sup-90. In all the samples, the greatest inhibition zone was toward Trichophyton rubrum, and the narrowest zone was related to Microsporum gypseum.
4.4. Determination of Minimum Inhibitory Concentrations
Minimum inhibitory concentrations of Sup-0 and LPE were tabulated in Table 1. It was found that the MIC of LPE for Trichophyton rubrum, Trichophyton verocosum, Microsporum canis and Microsporum gypseum was 0.625 mg/mL and the MIC of Sup-0 for these fungi was 6.25 mg/mL.
| Indicator Pathogens | Trichophytum rubrum | Trichophytum verrucosum | Microsporum canis | Microsporum gypseum |
|---|---|---|---|---|
| Concentration range of Sup-0, mg/mL | ||||
| 25 | - | - | - | - |
| 12.5 | - | - | - | - |
| 6.25 | - | - | - | - |
| 3.125 | + | + | + | + |
| 1.56 | + | + | + | + |
| Concentration Range of LPE, mg/mL | ||||
| 2.5 | - | - | - | - |
| 1.25 | - | - | - | - |
| 0.625 | - | - | - | - |
| 0.312 | + | + | + | + |
| 0.156 | + | + | + | + |
Minimum Inhibitory Concentrations of Fresh Supernatant (Sup-0) and Lyophilized Probiotic Extract
4.5. Different Gel Formulations
F1, F2 and F3 were chosen as the best formulations. F4, F5 and F6 were eliminated because of their very low viscosity, and F7, F8 and F9 formulations were eliminated because of their high viscosity (Table 2).
| Ingredients | Formulation of Gels | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | |
| HPMC | 2.5 | 2.5 | 2.5 | 2 | 2 | 2 | 3 | 3 | 3 |
| Distilled Water | 97 | 96 | 95 | 97 | 96 | 95 | 97 | 96 | 95 |
Different Gel-Based Formulations
4.6. Physicochemical Evaluation of Gel
The result for pH, viscosity, homogeneity and grittiness were shown in Tables 3 - 5.
| Formulation Code | Cp, η (cp) × 106 | Mean ± SD | Storage Time | ||
|---|---|---|---|---|---|
| cp1 | cp2 | cp3 | |||
| F1 | |||||
| 14.9 | 15 | 15.2 | 15.03 ± 0.15 | After a week | |
| 14.8 | 14.7 | 14.5 | 14.66 ± 0.15 | After a month | |
| 14.7 | 14.5 | 14.4 | 14.53 ± 0.15 | After 3 months | |
| F2 | |||||
| 14.5 | 14.6 | 14.7 | 14.6 ± 0.1 | After a week | |
| 14.5 | 14.3 | 14.4 | 14.4 ± 0.1 | After a month | |
| 14.4 | 14.1 | 14.2 | 14.23 ± 0.15 | After 3 months | |
| F3 | |||||
| 14 | 14.1 | 14.3 | 14.13 ± 0.15 | After a week | |
| 13.9 | 14 | 14.2 | 14.03 ± 0.15 | After a month | |
| 13.9 | 14 | 14.1 | 14 ± 0.1 | After 3 months | |
Evaluation Data of Viscosity of Lyophilized Probiotic Extract Gel
| Formulation Code | pH | Mean ± SD | Storage Time | ||
|---|---|---|---|---|---|
| pH1 | pH2 | pH3 | |||
| F1 | |||||
| 5.48 | 5.48 | 5.48 | 5.48 ± 0 | After 48 hours | |
| 5.38 | 5.38 | 5.39 | 5.38 ± 0.005 | After a week | |
| 5.39 | 5.38 | 5.40 | 5.39 ± 0.01 | After a month | |
| 5.39 | 5.38 | 5.38 | 5.38 ± 0.005 | After 3 months | |
| F2 | |||||
| 5.39 | 5.40 | 5.41 | 5.4 ± 0.01 | After 48 hours | |
| 5.30 | 5.31 | 5.30 | 5.30 ± 0.005 | After a week | |
| 5.30 | 5.32 | 5.31 | 5.31 ± 0.01 | After a month | |
| 5.31 | 5.30 | 5.31 | 5.30 ± 0.005 | After 3 months | |
| F3 | |||||
| 5.32 | 5.33 | 5.33 | 5.32 ± 0.005 | After 48 hours | |
| 5.27 | 5.28 | 5.29 | 5.28 ± 0.01 | After a week | |
| 5.22 | 5.22 | 5.23 | 5.22 ± 0.005 | After a month | |
| 5.21 | 5.21 | 5.22 | 5.21 ± 0.005 | After 3 months | |
Evaluation Data of pH of Lyophilized Probiotic Extract Gel
| Formulation | Homogeneity | Grittiness |
|---|---|---|
| F1 | +++ | - |
| F2 | +++ | - |
| F3 | +++ | - |
Evaluation Data of Homogeneity and Grittiness
4.7. Stability of Gel
The appearance of formulated gel remained clear and no significant change was observed in stability after a 3-month period.
4.8. Antidermatophytosis Effect of the Lyophilized Probiotic Extract Gel
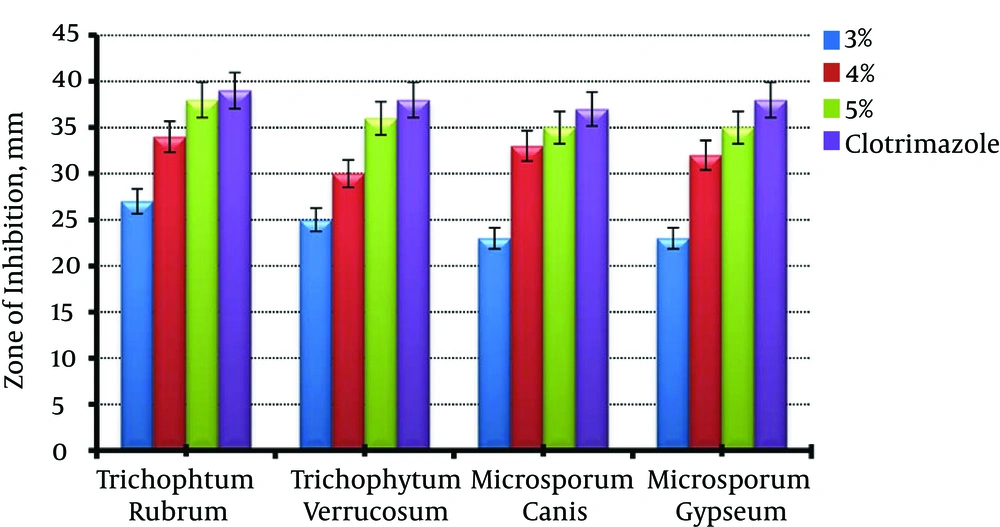
Figure 4 shows antifungal effect of different gel formulations. It was related to the content of LPE as formula contained 5% of LPE had a greatest inhibition zone. Also, there was no significant difference between 5% LPE gel and clotrimazole cream (P > 0.05).
5. Discussion
It has been shown that 20% to 30% of human infections are due to dermatophytes and there are several reports indicating their resistance to common antifungal agents. In the other hand, the commonly used antifungal agents including “azol” and “alylamine” compounds such as itraconazole and terbinafine are hepatotoxic and they cause many drug interactions (7).
In recent years, to overcome the difficulties due to dermal infections, many researchers have been focused on discovering new efficient topical agents with natural origin and fewer side effects. In 2010, Karska-Wysocki et al. investigated antimicrobial effect of Lactobacillus acidophilus and Lactobacillus casei on methicillin resistant Staphylococcus aureus (MRSA). They showed that Lactobacillus acidophilus produces antimicrobial compounds that inhibit MRSA growth to the extent that after 24 hours incubation of probiotic bacteria and pathogens at 37°C, there was 99% decrease in pathogens population. Also, Lactobacillus casei by producing different compounds such as lactic acid has bactericidal effect on MRSA pathogens (25). In 2012, Guo et al. studied the anti-fungal effect of 4 Lactobacillus types against Microsporum canis, Microsporum gypseum and Epidermophyton flocossum by the agar diffusion method. According to their findings, Lactobacillus reutei had the maximum antifungal effect in comparison to the other types (26).
Our findings showed that the maximum acid production was 18 to 24 hours after cultivation of the bacteria. Also, after a 9-month period, stability study showed about 0.3% to 0.35% increases in pH, which had a significant impact on antipathogenic efficacy of the extract. Therefore, to enhance the probiotic supernatant stability and also to concentrate and reinforce its efficacy, lyophilization was utilized as a proper method. According to our findings, LPE, in comparison with supernatant was more stable, and also had more pathogenic effect. Well-diffusion study results (Figure 3) demonstrated that aging may decrease the antifungal activity of total extract and due to equal antifungal efficacy of Sup-0 and probiotic, it may be utilized as a proper substitute. There was a significant difference between antidermatophytic efficacy of LPE and the supernatant (P < 0.05) and it showed the bigger growth inhibition zone in culture media. Also, as indicated in Table 1, LPE even in lower concentrations, was significantly more potent in growth inhibition than the plain probiotic extract (P <0.01). Macroscopic evaluation of LPE containing gel showed its homogeneity and there were no rigidity and air bubbles (Table 5). Also, there was no considerable pH change during three months of storage (Table 3) and it can be deduced that by increasing the extract content of the gels, the formulation becomes more acidic.
Rheological evaluation showed no significant change in gel viscosities, although it was an inverse relation between LPE content and viscosity (Table 4). Figure 4 shows an antifungal effect of different gel formulations. It can be seen that all of the formulations inhibit growth of common dermatophytes, although 5% LPE gel was significantly more effective (P < 0.05). Also, there was no significant difference between 5% LPE gel and clotrimazole cream (P > 0.05). Therefore, due to safety and lack of adverse effects of probiotic containing products, they may be considered as a proper substitute in the treatment of dermatophytosis.
From this study, it can be concluded that the prepared probiotic topical gel, due to its efficacy and safety can be used for the treatment of dermatophytosis. However, it is needed for in vivo application to examine clinically the prepared gel containing LPE in more detail.