1. Background
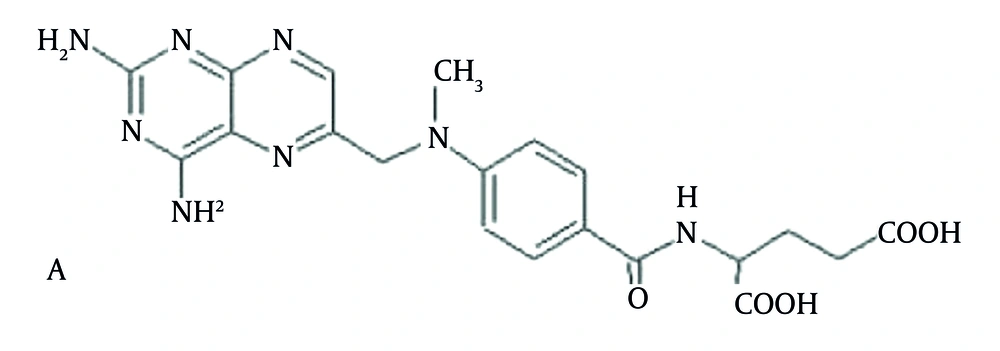
Methotrexate (MTX) (Figure 1) is a folic acid antagonist that has been in clinical use for five decades. Its use in patients with brain tumors is primarily confined to primary central nervous system lymphoma (PCNSL), in which it is the cornerstone of chemotherapy (1). In addition to the clinical use of MTX, it has been the subject of many preclinical investigations pertaining to its interesting pharmacological properties. One area of interest pertinent to its use in PCNSL is the cellular mechanisms that control the uptake and accumulation of MTX in the central nervous system (CNS). MTX is a substrate for certain members of the ABC transporter family, which serve as drug efflux pumps that can alter MTX’s pharmacokinetic properties and the associated sensitivity of tumor cells (2-4). As some of these transporters are located on the blood-brain barrier (BBB) and the blood-cerebrospinal fluid barrier (BCSFB), which often limit drug accumulation in the brain (5, 6), they may also influence MTX’s CNS accumulation. In addition to these anatomic barriers, drug efflux pumps also operate in tumor cells and may be a contributing factor to a drug-resistance phenotype due to their ability to limit the drug’s access to the intracellular space (7). The pharmacokinetics of MTX are characterized by large inter-individual variability. This can be an important factor influencing both clinical outcomes and the risk of MTX toxicity. The pharmacokinetic characteristics have been shown to be good predictors of the anti-psoriatic effect of MTX. Thus, to facilitate the exploration of the determinants of MTX’s CNS distribution in preclinical tumor models, we developed a sensitive liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS) method based on the use of small sample volumes. Due to the long history of MTX as an anticancer drug, there have been numerous high-performance liquid chromatography (HPLC), spectrofluorimetry, and capillary electrophoresis-based methods to quantitate MTX in biological specimens (8, 9). Most often, these methods utilize solid-phase extraction for sample clean-up with either UV or derivative fluorescence detection (10-15). Since most of these methods were developed for human samples, relatively large volumes (i.e. > 0.5 mL) of plasma were needed to achieve the desired sensitivity limit. There are a limited number of methods reported for the quantitation of MTX in preclinical samples (16, 17). Measurement of MTX in human plasma by LC-MS/MS has yielded improved sensitivity limits to 0.5 ng/mL, based upon a 200 μL plasma sample and a liquid-liquid extraction procedure, and to 5 ng/mL, based upon 20 μL of plasma and a solid-phase extraction procedure (18). Previous works measured MTX with the HPLC and LC-MS methods in human plasma (15, 19-22).
2. Objectives
In the present study, we describe a simple, selective, and stable method using HPLC coupled with EIS and quadrupole mass spectrometry for the determination of MTX in human plasma.
3. Materials and Methods
3.1. Materials
MTX extended-release test tablets (batch no. 014, Zahravi), MTX reference tablets (batch no. 801892,), and an MTX reference standard (99.9% purity) were supplied and identified by Ebewe Pharma (Vienna, Austria). Acetonitrile was HPLC-grade and was purchased from Merck (Germany). Plasma was obtained from the Iranian blood transfusion organization. Other chemicals and solvents were of analytical or chemical lab purity grades as needed, and were purchased from Emertat Shimi (Iran).
3.2. Instrumentation and Operating Conditions
3.2.1. Liquid Chromatography
Liquid chromatography was carried out using an HPLC system (Agilent Technologies, model LC-1200, Englewood, CA, USA) equipped with an auto-sampler. The analytical column used was a C18 column (ZORBAX XDB-ODS, USA) (2.1 × 100 mm, 3.5 micron), operated at 25°C. The mobile phase consisted of acetonitrile/water/formic acid 74:25:1 (v/v), set at a flow rate of 0.2 mL/minutes.
3.2.2. Mass Spectrometry
Mass spectrometric (MS) detection was performed using a triple-quadrupole mass spectrometer (Agilent Technologies, model LCMS-6410, Englewood, CA, USA) with an ESI interface. The ESI source was set at positive ionization mode. The formula [(M + H) + m/z 455.3] for MTX was selected for detecting ions. The MS operating conditions were optimized as follows: ion spray voltage was set to 4000 V, the temperature of the ion transfer capillary was 250°C, nebulizer gas (NEB) was 10, and curtain gas (CUR) was 8. The quantification was performed via peak-area. Data acquisition and processing were accomplished using Agilent LCMS Solution software for the LCMS-6410 system.
3.3. Preparation of Stock Solutions
Stock solutions of MTX were prepared in the HPLC mobile phase at concentrations of 1 mg/mL and were stored at 4°C. Working solutions of MTX were prepared daily in the HPLC mobile phase by appropriate dilutions at 5.0, 10.0, 25.0, 50.0, 100.0, 250.0, 500.0, 1000, and 1500 ng/mL.
3.4. Sample Preparation and Extraction Procedure
A 0.1 mL aliquot of a plasma sample from a human volunteer was pipetted into a 1 mL centrifuge tube, then 0.1 mL of acetonitrile was added and the sample was vortexed for 2 minutes. After centrifugation (Heidolph, Germany) of the sample at 15,400 rpm for 20 minutes, the organic layer was transferred to another 1 mL centrifuge tube and an aliquot of 20 µL was injected into the LC-MS system.
3.5. Standard Curves
Proper volumes of one of the above-mentioned working solutions were used to produce the standard curve point’s equivalent to 0.1, 0.20, 1.0, 5.0, 10.0, 20.0, 50.0, and 100.0 ng/mL of MTX, and each sample was processed as described. Finally, the nominal known plasma concentrations were plotted against the corresponding peak areas to construct the standard curve.
3.6. Preparation of Quality-Control Samples
Quality control (QC) samples were prepared daily by spiking different samples of 0.1 mL of each plasma sample with the proper volume of the corresponding standard solution to produce a final concentration equivalent to a low level (0.1 ng/mL), middle level (20.0 ng/mL), and high level (100.0 ng/mL) of MTX. The procedure was the same as described above.
3.7. Method Validation
The method was validated for selectivity, linearity, accuracy, precision, recovery, stability, detection limit, and quantitation limit according to the industry guidance principles of the United States’ food and drug administration (FDA) (23).
3.7.1. Assay Specificity
To evaluate the matrix effect on the ionization of analytes, five different concentration levels of MTX (0.1, 0.20, 5.00, 10.00, and 100.00 ng mL-1) were prepared in the drug-free blank plasma as a five-sample series using different lots of the drug-free plasma. The samples were processed as described, and injected into LC-MS. The same concentrations were prepared in the mobile phase instead of plasma and analyzed for drug concentrations using the same procedure. A comparison of the matrix effects of the two variants was made as an indicator of the method’s specificity.
3.7.2. Linearity
Standard curves of ten concentrations of MTX in a range of 0.1 - 100.0 ng/mL were assayed. Blank plasma samples were analyzed to ensure the lack of interference, but were not used to construct the calibration function. The limit of detection (LOD) was estimated from the signal-to-noise ratio. This parameter was defined as the lowest concentration level resulting in a peak area of three times the baseline noise. The limit of quantification (LOQ) was defined as the lowest concentration level that provided a peak area with a signal-to-noise ratio higher than 5, with precision (% CV) within ± 20% and accuracy (% recovery) between 80% and 120% (24, 25).
3.7.3. Precision and Accuracy
3.7.3.1. Within-Run Variations
In one run, three samples with concentrations of 0.2, 20, and 100 ng/mL (from the high, middle, and low regions of the standard curve, respectively) were prepared in triplicate and analyzed by the developed LC-MS method. The coefficient of variations (CV%) of the corresponding determined concentrations were then calculated in each case.
3.7.3.2. Between-Run Variations
On three different runs, samples from the upper, intermediate, and lower concentration regions used for the construction of the standard curve (identical to the within-run variations test) were prepared and analyzed with the LC-MS method. The corresponding CV% values were then calculated.
3.7.3.3. Repeatability Test
To test the method’s repeatability, six independent spiked plasma samples with a drug concentration of 5 ng/mL were prepared as described. A single injection of each preparation was made into LC-MS and the %RSD between the results was determined as the repeatability of the method.
3.7.4. Extraction Recovery
Three samples with concentrations of 0.2, 20, and 100 ng/mL (from the high, middle, and low regions of the standard curve, respectively) were prepared in triplicate and analyzed with the developed LC-MS method. The ratio of the recorded peak heights to the peak heights resulting from the direct injection of the aqueous solutions of MTX with the same concentrations were then determined as percentages in each case.
3.7.5. Intermediate Precision
On a different day to that of the repeatability study, a second researcher executed an analysis of six further samples prepared as described in the repeatability test procedure. The analysis was carried out using fresh reagents and a different HPLC column. The %RSD between the six measurements was determined along with the %RSD between the total of 12 measurements from the repeatability and intermediate precision tests.
3.7.6. Reproducibility
The mean results for the same sample analysis between our laboratory and two different test facilities were obtained, and the % difference between content measurements was calculated using the Equation 1:

3.7.7. Stability
3.7.7.1. Freeze and Thaw Stability
Three concentration levels of QC plasma samples were stored at -20°C for 24 hours and thawed unassisted at room temperature. When completely thawed, the samples were refrozen for 24 hours under the same conditions. The freeze-thaw cycle was repeated twice, and the samples were tested after three cycles (26, 27).
3.7.7.2. Short-Term Temperature Stability
Three concentration levels of QC plasma samples were kept at room temperature for a period that exceeded the routine preparation time of the samples (approximately 6 hours).
3.7.7.3. Long-Term Stability
Three concentration levels of QC plasma samples kept at low temperature (-20°C) were studied for a period of 4 weeks.
3.7.7.4. Post-Preparative Stability
The auto-sampler stability was checked by reanalyzing the extracted QC samples kept under the auto-sampler conditions (4°C) for 12 hours.
3.8. Clinical Study Design
Twelve male subjects were enrolled in a randomized, two-treatment, two-period, single-dose crossover study with a weeklong washout between the first dose of period I and the first dose of period II. The single-dose study subjects fasted from the night before dosing until 2 hours after dosing for each session. For the MTX reference group (Ebewe pharmaceutical, batch no. 801892; Vienna, Austria), the 10 mg MTX formulation was administered, and blood samples were obtained prior to dose administration (time 0) and at 0.25, 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, 6.0, 8.0, 10.0, 24.0, and 48.0 hours after the dose. For the MTX test group (batch No. 014, Zahravi), the 10 mg MTX test formulation was administered, and blood samples were obtained prior to dose administration (time 0) and at 0.25, 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, 6.0, 8.0, 10.0, 24.0, and 48.0 h after the dose. The blood samples were immediately centrifuged at 1600 g for 10 minutes. The plasma was removed and stored at -20°C until analysis.
3.8.1. Pharmacokinetic Study
The pharmacokinetic parameters for MTX were designed using standard non-compartmental methods. The peak serum concentration (Cmax) and the time to reach it (Tmax) were evaluated by a visual examination of the data and used as criteria of the rate of absorption. The apparent elimination rate constant (β) was determined by linear regression of log-transformed data in the terminal phase of the serum concentration-time profile (28). The elimination half-life (t1/2) was considered the quotient of 0.693/β. In addition, the area under the curve for plasma concentration time (AUC0-t) was determined by the linear trapezoidal rule from the measured serum concentrations from zero to the time of the last quantifiable concentration (Ct). The AUC0-∞, the area under the serum concentration-time curve extrapolated to perpetuity, was designed according to the following equation: AUC0-∞ = AUC0-t + Ct/Kel. The pharmacokinetic profiles of MTX from the two tablet formulations were compared, and the comparative bioavailability of the test/reference products was calculated using the ratio of AUC0-∞ (test) / AUC0-∞ (reference). The pharmacokinetic parameters were statistically compared by analysis of variance (ANOVA) to evaluate the consequence of formulations. The 90% confidence intervals were constructed for the ratio of the income of the test and reference products and were compared to the reference intervals (0.8 - 1.20) as recommended by the FDA (29).
4. Results
4.1. Method Development
Considering the complex biological matrix of the samples to be analyzed and the nature of the method to be used for the drug assay, the method-development efforts focused on two different areas of sample preparation and analyte separation, which are discussed in detail in the following sections.
4.1.1. Sample Preparation
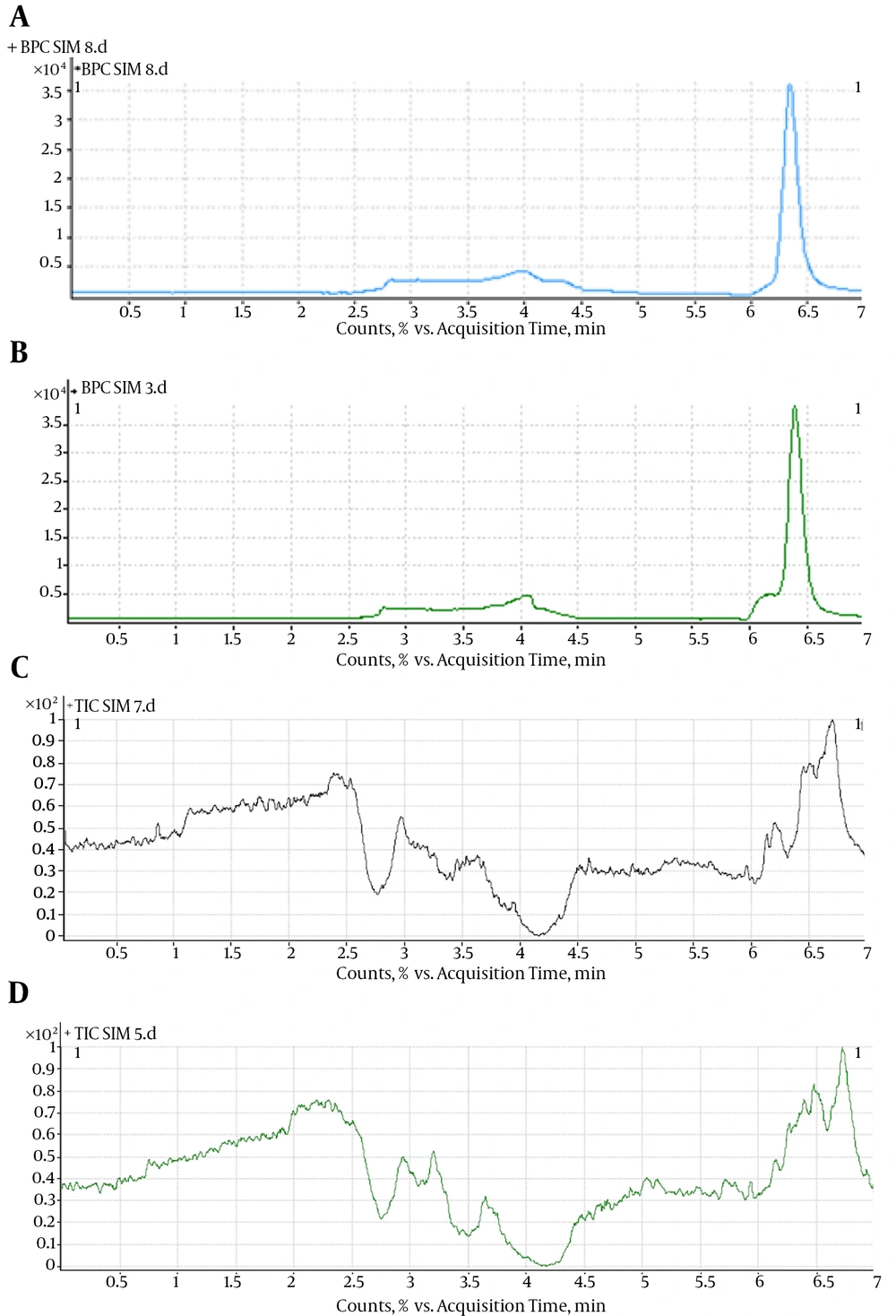
Methanol, perchloric acid, and acetonitrile were all attempted, and acetonitrile was finally adopted because of its high extraction efficiency and lower interference. Precipitation with and without adding 0.1 M NaOH (100 µL) was tried, and obvious differences were not observed, so the precipitation using acetonitrile without adding 0.1 M NaOH was finally used. The chromatograms for plasma sample (MTX concentration = 4 ng/mL) extraction by different solvents are shown in Figure 2.
4.1.2. Analyte Separation
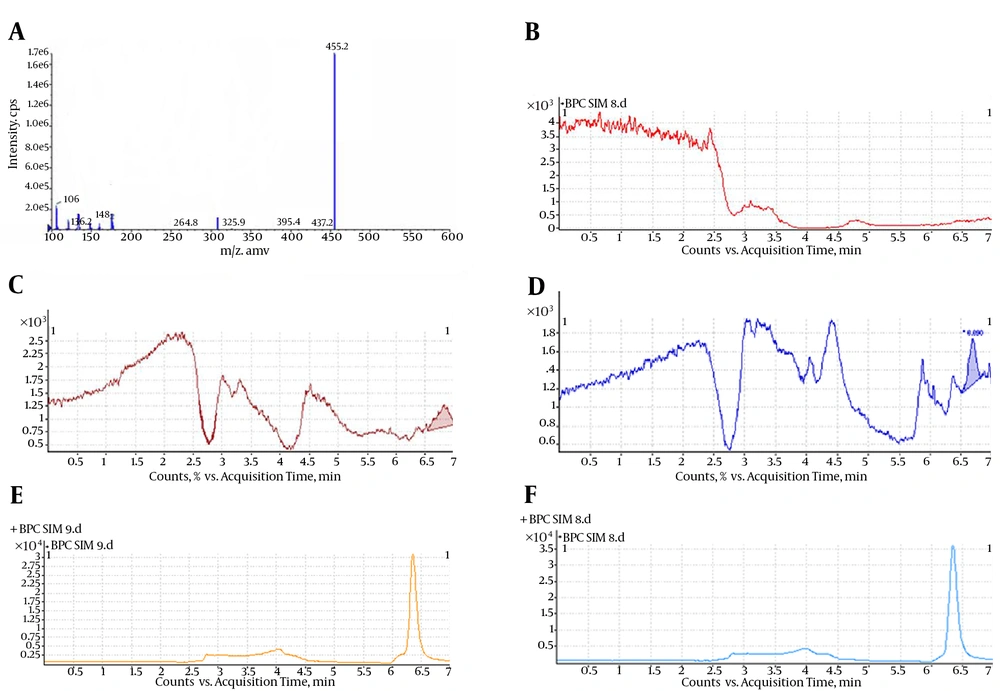
LC-MS/MS with positive ESI was selected to detect MTX in human plasma. A prominent fragment with m/z 455.3 was observed on the product ion scan with positive ESI (Figure 3A). According to the mass scan spectrum, the m/z of 455.3, produced by the quasi-molecule ion [M+H]+ of MTX, was selected for monitoring. The selected-ion monitoring (SIM) (+) chromatograms extracted from the supplemented plasma are depicted in Figure 3. The retention time of MTX was 6.5 minutes. The total HPLC-MS analysis time was 7 minutes per sample. A representative chromatogram of a plasma sample obtained at 6 hours from a subject who received a single oral dose (10 mg) is shown in Figure 3E. No interference was observed. Figure 3B shows an HPLC chromatogram for a blank plasma sample, indicating no endogenous peaks at the retention positions of MTX.
A, positive ion electrospray mass scan spectrum of MTX; B, blank plasma; C, LOD (MTX concentration = 0.05 ng/mL); D, LOQ (MTX concentration = 0.10 ng/mL); E, SIM (+) chromatograms for plasma sample of a healthy volunteer (MTX concentration = 5.12 ng/mL); F, supplemented plasma (MTX concentration = 4 ng/mL).
4.2. Method Validation
4.2.1. Assay Specificity
It is clearly evident from the typical chromatograms of the developed method shown in Figure 3 that there are no discernible interferences between the matrix factors and the analyte. This, in turn, ensures obtaining reliable results with this method for determining the biological concentrations of MTX.
4.2.2. Linearity and LOQ
The present method produced linear responses throughout the MTX concentration range of 0.1 - 100 ng/mL, which is suitable for the intended purposes. A typical linear regression equation of the method was: y = 36689x + 49747, with x and y representing MTX concentration (in ng/mL) and peak area (in arbitrary units), respectively, with a regression coefficient (r) of 0.998. The lower limit of quantification for MTX was proved to be 0.10 ng/mL and the limit of detection was 0.05 ng/mL. Figure 3C shows the chromatogram of an extracted sample that contained 0.05 ng/mL (LOD) of MTX. Figure 3D shows the chromatogram of an extracted sample that contained 0.10 ng/mL (LOQ) of MTX.
4.2.3. Precision and Accuracy
4.2.3.1. Within-Run Variations and Accuracy
The within-run variations of the developed LC-MS method, as well as the corresponding absolute recoveries, are shown in Table 1. These data clearly show that the developed method has an acceptable degree of repeatability and accuracy within an analytical run.
| Nominal Added Concentration, ng/mL/Sample Number | Measured Concentration, ng/mL | Mean (SD) | CV% |
|---|---|---|---|
| 0.1 | 0.10 (0.01) | 11 | |
| 1 | 0.12 | ||
| 2 | 0.08 | ||
| 3 | 0.10 | ||
| 20 | 20.03 (0.06) | 0.32 | |
| 1 | 20.03 | ||
| 2 | 19.97 | ||
| 3 | 20.1 | ||
| 100 | 99.46 (0.81) | 0.82 | |
| 1 | 100.40 | ||
| 2 | 98.93 | ||
| 3 | 99.05 |
Within-Run Variations and Accuracy of the LC-MS Method for Quantitation of MTX (n = 3)
4.2.3.2. Between-Run Variations and Accuracy
The between-run variations of the developed LC-MS method, as well as the corresponding absolute recoveries, are shown in Table 2. As stated for the previous test, these data clearly show that the developed method has an acceptable degree of reproducibility and accuracy between different analytical runs.
| Nominal added concentration, ng/mL/Run Number | Measured Concentration, ng/mL | Mean (SD) | CV% |
|---|---|---|---|
| 0.1 | 0.1 (0.01) | 10 | |
| 1 | 0.10 | ||
| 2 | 0.11 | ||
| 3 | 0.09 | ||
| 20 | 20.2 (0.96) | 4.62 | |
| 1 | 20.07 | ||
| 2 | 20.93 | ||
| 3 | 19.08 | ||
| 100 | 99.65 (0.58) | 0.58 | |
| 1 | 99.01 | ||
| 2 | 99.80 | ||
| 3 | 100.15 |
Between-Run Variations and Accuracy of the LC-MS Method for Quantitation of MTX (n = 3)
4.2.3.3. Repeatability Test
The repeatability of the method is shown in Table 3, which demonstrates a remarkable repeatability for the drug assay in plasma.
| Sample | Peak Area | Retention Time, min |
|---|---|---|
| 1 | 2435432 | 6.54 |
| 2 | 2431234 | 6.71 |
| 3 | 2436578 | 6.77 |
| 4 | 2432321 | 6.59 |
| 5 | 2439087 | 6.58 |
| 6 | 2438765 | 6.65 |
| Mean (SD) | 2435570 (3254.82) | 6.64 (0.08) |
| CV% | 0.133 | 1.31 |
Repeatability of Test Results for Spiked Plasma Containing 5 ng mL-1 MTX
4.2.4. Relative Recovery (Matrix Effect)
The extraction recovery determined for MTX was shown to be consistent, precise, and reproducible. The data are shown in Table 4 indicate that there was no significant matrix effect on the outputs of the assay method.
| Nominal Added Concentration ng/mL, Sample Number | CV% | Mean (SD) | Percent Recovery, % |
|---|---|---|---|
| 0.1 | 95.78 (2.47) | 2.58 | |
| 1 | 98.15 | ||
| 2 | 93.21 | ||
| 3 | 95.98 | ||
| 20 | 94.67 (1.58) | 1.67 | |
| 1 | 93.42 | ||
| 2 | 96.45 | ||
| 3 | 94.15 | ||
| 100 | 93.47 (1.80) | 1.93 | |
| 1 | 91.60 | ||
| 2 | 95.21 | ||
| 3 | 93.61 |
Relative Recovery of MTX by the LC-MS Method (n = 3)
4.2.5. Intermediate Precision
The results of the intermediate precision test are shown in Table 5. As indicated, the developed method shows an acceptable intermediate precision for MTX assaying.
| Sample | Peak Area | Retention time, min |
|---|---|---|
| 1 | 2435621 | 6.73 |
| 2 | 2431234 | 6.55 |
| 3 | 2435467 | 6.65 |
| 4 | 2439087 | 6.71 |
| 5 | 2437689 | 6.68 |
| 6 | 2431327 | 6.59 |
| Mean (SD) | 2435071 (3230.52) | 6.65 (0.06) |
| CV% | 0.132 | 1.05 |
Intermediate Precision of the Test Results for Spiked Plasma Containing 5 ng mL-1
4.2.6. Reproducibility
The highest test result of the spiked plasma with 5 ng mL-1 MTX was 2,439,087 and the lowest value was 2,431,234, with a mean value of 2,435,071. Therefore, the % difference was 0.32%, indicating high reproducibility for this method.
4.2.7. Stability
Table 6 summarizes the freeze-and-thaw stability, short-term stability, long-term stability, and post-preparative stability data of MTX. All of the results showed stable behavior during these tests, and there were no stability-related problems during the routine sample analysis for pharmacokinetic, bioavailability, or bioequivalence studies. The stability of the working solutions was tested at room temperature for 6 hours based on the results obtained; these working solutions were stable within 6 hours.
| Stability | 0.2, ng/mL | SD | 20, ng/mL | SD | 100, ng/mL | SD |
|---|---|---|---|---|---|---|
| Short-term stability | 91.98 | 1.2 | 95.43 | 0.91 | 95.09 | 0.87 |
| Freeze and thaw stability | 94.42 | 0.23 | 97.14 | 2.1 | 93.76 | 1.2 |
| Long-term stability | 95.66 | 1.01 | 92.87 | 0.91 | 93.89 | 1.13 |
| Post-preparative stability | 92.87 | 0.76 | 91.87 | 1.34 | 91.81 | 0.34 |
Data Showing Stability of MTX in Human Plasma at Different QC Levels (n = 5)a
4.3. Bioequivalence Study
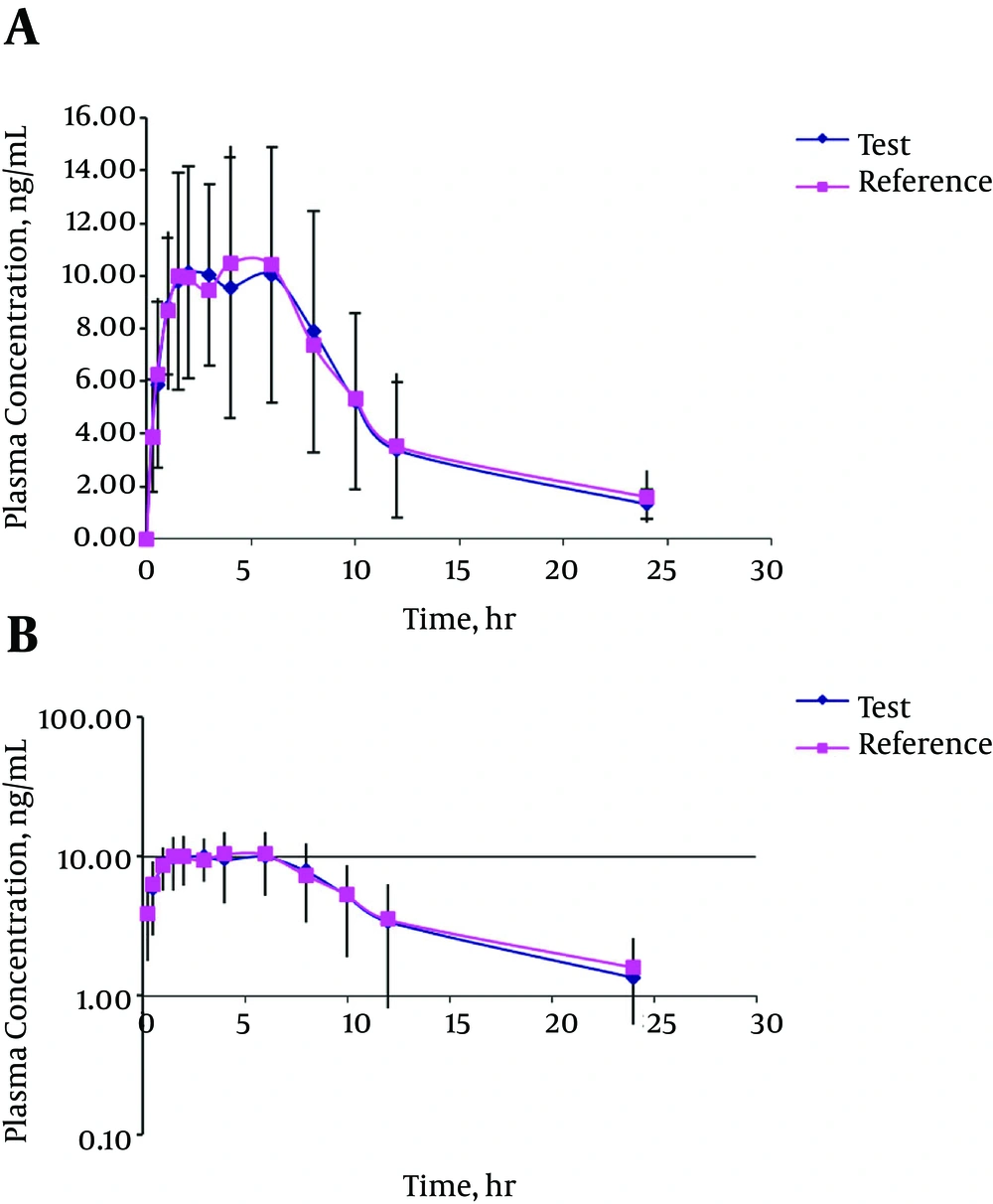
The mean serum concentration-time profiles after single-dose oral administration of the reference and test formulations are shown in Figure 4. The mean serum concentration-time curves from the two test and reference products were nearly superimposable. Furthermore, there was no important distinction between MTX serum concentrations at each time-point subsequent to oral administration of the two formulations. At the first case-time (0.5 hours), the drug was identifiable in all subjects following the administration of both formulations. The resulting pharmacokinetic parameters are shown in Table 7. Mean maximum serum concentrations of 13.94 ± 4.36 ng/mL and 13.49 ± 3.67 ng/mL were obtained for the test and reference formulations, respectively. Tmax, the time required to reach the maximum serum concentration, was 2.63 ± 1.45 hours and 2.75±1.74 hours, respectively. In addition to Cmax and Tmax, the ratio of Cmax/AUC0-∞ can be used as a parameter for determining the absorption rates in bioequivalence studies (30, 31). These calculated ratios were 9.90% and 10.71% for the test and reference formulations, respectively. The parameters used for the amount of absorption were AUC0-t, AUC0-∞. The AUC0-t and AUC0-∞ for the test formulation were 122.57 ± 54.34 ngh/mL and 140.74 ± 56.69 ngh/mL, respectively. The considered values for the reference formulation were 125.94 ± 53.09 ngh/mL and 155.80 ± 65.11 ng h/mL, in the order mentioned. The confidence limits shown in Table 8 reveal that these values are completely within the acceptable bioequivalence range of 80% - 120% as set by the FDA and EMEA (29). The multivariate analysis proficiency through ANOVA indicated that there were no statistically significant differences between the two formulations within any of the pharmacokinetic parameters. Furthermore, the periods and sequence property did not influence the outcome of the statistical analysis. Both of the formulations were well-tolerated by all volunteers in both phases of the study, with no clinical adverse events. All calculated pharmacokinetic parameter values were in good agreement with the previously reported values (32, 33). For the bioequivalence evaluation, Cmax, AUC0-t, and AUC0–∞ were considered as primary parameters. The mean and standard deviation of these parameters of the two formulations were found to be very close, indicating that the plasma profiles generated by the test formulation were comparable to those of the reference formulation.
| Volunteer Code | PK Parameter | |||||||
|---|---|---|---|---|---|---|---|---|
| Cmax, µg/mL | tmax, h | AUC0-8, µgh/mL | AUC0-∞, µg.h/mL | |||||
| Test | Reference | Test | Reference | Test | Reference | Test | Reference | |
| 1 | 12.88 | 13.86 | 3.00 | 3.00 | 87.86 | 84.23 | 97.24 | 91.05 |
| 2 | 9.53 | 9.79 | 1.00 | 6.00 | 100.03 | 109.81 | 111.00 | 123.60 |
| 3 | 19.65 | 14.81 | 1.50 | 1.50 | 101.17 | 99.50 | 112.97 | 110.61 |
| 4 | 8.68 | 9.28 | 2.00 | 1.50 | 79.51 | 88.41 | 88.43 | 109.18 |
| 5 | 7.98 | 9.30 | 1.50 | 1.50 | 78.05 | 82.76 | 97.91 | 96.73 |
| 6 | 10.33 | 12.17 | 1.50 | 1.50 | 67.72 | 70.52 | 83.29 | 101.78 |
| 7 | 14.23 | 14.63 | 6.00 | 1.00 | 119.83 | 106.56 | 144.71 | 209.54 |
| 8 | 12.16 | 9.02 | 3.00 | 3.00 | 108.59 | 118.30 | 159.98 | 130.34 |
| 9 | 18.76 | 17.50 | 2.00 | 2.00 | 113.30 | 147.38 | 128.99 | 211.79 |
| 10 | 19.15 | 19.36 | 4.00 | 6.00 | 237.33 | 248.67 | 258.97 | 299.22 |
| 11 | 18.98 | 18.37 | 4.00 | 4.00 | 213.78 | 195.88 | 232.36 | 212.71 |
| 12 | 14.92 | 13.80 | 2.00 | 2.00 | 163.67 | 159.28 | 173.02 | 173.10 |
| Mean | 13.94 | 13.49 | 2.63 | 2.75 | 122.57 | 125.94 | 140.74 | 155.80 |
| SD | 4.36 | 3.67 | 1.45 | 1.74 | 54.34 | 53.09 | 56.69 | 65.11 |
| CV% | 31.30 | 27.22 | 55.16 | 63.22 | 44.33 | 42.15 | 40.28 | 41.79 |
| Max | 19.65 | 19.36 | 6.00 | 6.00 | 237.33 | 248.67 | 258.97 | 299.22 |
| Min | 7.98 | 9.02 | 1.00 | 1.00 | 67.72 | 70.52 | 83.29 | 91.05 |
| P value | 0.79 | 0.79 | 0.85 | 0.85 | 0.88 | 0.88 | 0.55 | 0.55 |
The Mean Primary Pharmacokinetic Parameters of MTX in 12 Healthy Volunteers Following Oral Administration of 10 mg of Test and Reference Products
| Volunteer Code | PK Parameter | |||||||
|---|---|---|---|---|---|---|---|---|
| Λz,h-1 | MRT, h | Cl, L/h | Vd,ss, L | |||||
| Test | Reference | Test | Reference | Test | Reference | Test | Reference | |
| 1 | 0.090 | 0.077 | 9.65 | 7.87 | 41.13 | 43.93 | 396.79 | 345.84 |
| 2 | 0.097 | 0.087 | 10.23 | 10.84 | 36.04 | 32.36 | 368.73 | 350.70 |
| 3 | 0.067 | 0.068 | 9.47 | 9.27 | 35.41 | 36.16 | 335.48 | 335.19 |
| 4 | 0.086 | 0.059 | 10.13 | 14.09 | 45.23 | 36.64 | 458.31 | 516.05 |
| 5 | 0.070 | 0.079 | 14.92 | 12.16 | 40.85 | 41.35 | 609.39 | 503.03 |
| 6 | 0.053 | 0.035 | 13.75 | 21.27 | 48.03 | 39.30 | 660.48 | 836.10 |
| 7 | 0.058 | 0.015 | 13.12 | 48.74 | 27.64 | 19.09 | 362.61 | 930.47 |
| 8 | 0.037 | 0.108 | 21.71 | 10.60 | 25.00 | 30.69 | 542.86 | 325.44 |
| 9 | 0.076 | 0.050 | 10.42 | 19.59 | 31.01 | 18.89 | 323.04 | 370.02 |
| 10 | 0.112 | 0.077 | 10.55 | 13.89 | 15.45 | 13.37 | 162.94 | 185.69 |
| 11 | 0.115 | 0.113 | 10.42 | 10.28 | 17.21 | 18.80 | 179.31 | 193.30 |
| 12 | 0.131 | 0.112 | 9.06 | 9.86 | 23.12 | 23.11 | 209.52 | 227.75 |
| Mean | 0.083 | 0.073 | 11.95 | 15.71 | 32.18 | 29.47 | 384.12 | 426.63 |
| SD | 0.028 | 0.030 | 3.59 | 11.17 | 10.71 | 10.38 | 161.28 | 237.70 |
| CV% | 33.75 | 41.44 | 29.99 | 71.11 | 33.28 | 35.23 | 41.99 | 55.71 |
| Max | 0.13 | 0.11 | 21.71 | 48.74 | 48.03 | 43.93 | 660.48 | 930.47 |
| Min | 0.04 | 0.01 | 9.06 | 7.87 | 15.45 | 13.37 | 162.94 | 185.69 |
| P value | 0.45 | 0.45 | 0.28 | 0.28 | 0.54 | 0.54 | 0.61 | 0.61 |
The Mean Secondary Pharmacokinetic Parameters of MTX in 12 Healthy Volunteers Following Oral Administration of 10 mg of Test and Reference Products
This study examined the pharmacokinetic properties, bioavailability, and bioequivalence of MTX test and reference products, using a newly developed dispersible tablet and an established branded tablet, in healthy Iranian volunteers. The Cl (L/hr) were completely contained within the predefined bioequivalence of the MTX test and reference products at 32.18 and 29.47, respectively, and the MRT (hr) for the MTX test and reference products were 11.95 and 15.71, respectively (Table 8). These results indicated that the analytical method was linear, precise, and accurate. The multivariate analysis proficiency through ANOVA indicated that there were no statistically significant differences between the two formulations within any of the pharmacokinetic parameters (Table 9). The test and reference formulations were found to be bioequivalent.
| Pharmacokinetics Parameters | ANOVA (P Value) | 90% Cl |
|---|---|---|
| Cmax | 0.79 | 87 - 110 |
| tmax | 0.85 | 96 - 112 |
| AUC0-24 | 0.88 | 87 - 101 |
| AUC0-∞ | 0.55 | 93 - 113 |
ANOVA Results
5. Discussion
MTX is a folic acid antagonist that has been in clinical use for five decades. Its use in patients with brain tumors is primarily confined to primary central nervous system lymphoma (PCNSL), in which it is the cornerstone of chemotherapy (1). In addition to the clinical use of MTX, it is the subject of many preclinical investigations due to to its interesting pharmacological properties. The pharmacokinetics of MTX are characterized by large inter-individual variability. This can be an important factor, influencing both clinical outcomes and the risk of MTX toxicity. Pharmacokinetic characteristics were shown to be good predictors of the anti-psoriatic effect of MTX. Thus, to facilitate the exploration of the determinants of MTX’s CNS distribution in preclinical tumor models, there are some LC-MS methods for the analysis of MTX in human plasma, including spectrofluorimetry and capillary electrophoresis-based methods to determine MTX in biological specimens (8, 9). Most often, these methods utilize solid-phase extraction for sample clean-up with either UV or derivative fluorescence detection (10-15). Since most of these methods were developed for human samples, relatively large volumes (i.e. > 0.5 mL) of plasma were needed to achieve the desired sensitivity limit. There are a limited number of methods reported for the quantitation of MTX in human plasma. However, some of these methods have shortcomings, including instability of samples, cost of agents, and rigidness encountered in sample preparation. In contrast, the LC-MS method used in this study was cheap, sensitive, quick, accurate, and easily available.
5.1. Conclusions
A sensitive, selective, accurate, and precise HPLC method with selected ion monitoring by a single quadrupole mass spectrometer with an ESI interface was developed and validated for determination of MTX in human plasma. This method offers several advantages, such as a rapid and simple extraction scheme and a short chromatographic run-time, which make it suitable for the analysis of large sample batches used for studying the pharmacokinetics, bioavailability, or bioequivalence of MTX formulations. This method was successfully applied to a bioequivalence study of text and reference tablets containing MTX in healthy Iranian male subjects.