1. Background
It is well known that the liver is intimately involved in biochemical activities in the human body (1). This organ plays a pivotal role in the metabolism and detoxification of constituents that enter the body (2). The liver regulates hemostasis (3) and appears to have the physiological function of protecting the body from harmful drugs and chemicals (3). Considering the high prevalence of liver disease globally (2), the pathogenesis of this disease is an important public health problem (4). Despite significant scientific advancements in the field of hepatology in recent years, the incidence of liver disease is still increasing (3). Cell damage caused by free radicals is the leading mechanism of hepatotoxicity (5).
Carbon tetrachloride (CCl4) is a common toxin used to induce liver injury (6) in experimental studies. Hepatic responses in rats to chronic CCl4 stimulation are superficially similar to human cirrhosis (7). The mechanism initiates lipid peroxidation and protein oxidation, which leads to liver damage (8).
In vitro and in vivo experiments have demonstrated that select herbs protect against oxidative damage by inhibiting or quenching free radicals and reactive oxygen species (ROS) (9). Pyrusbiossieriana is a species of pear that is native to Iran. Its leaves contain high levels of arbutin, a glycosylated hydroquinone that inhibits tyrosinase. This plant has a variety of pharmacological and therapeutic properties, including anti-inflammatory, antiviral, anti-hyperglycemic, anti-hyperlipidemic, and antioxidant activity (10). It has been shown to scavenge free radicals (11), be gastroprotective (12), and exhibit alpha-glycosidase and alpha-amylase inhibitory activity (13). Previous studies have revealed that plants containing polyphenols demonstrate clear anti-hepatotoxic properties (14).
2. Objectives
The present study investigated the antioxidant potential of arbutin on biochemical factors, CCl4-mediated oxidative stress, and hepatocyte dysfunction in CCl4-induced liver damage in rats.
3. Methods
3.1. Animals
For this study, 63 apparently healthy male Wistar rats (150 - 200 g) were obtained from the animal facility at Babol University of Medical Sciences in the city of Babol in Iran. They were housed in wire-floored cages under standard laboratory conditions with a 12-hour light/12-hour dark cycle at 25 ± 2°C and had access to a standard rat chow diet and water ad libitum. The experimental protocols were approved by the research committee of Babol University of Medical Sciences. This study was carried out in accordance with the guidelines of the institutional animal ethics committee.
3.2. Drugs and Chemicals
CCl4 was purchased from Merck (Germany). Thiobarbituric acid (TBA) and 2, 4, 6-tripyridyl-s-triazine (TPTZ) were obtained from Sigma Aldrich (US). Biochemical factors and enzymes were determined using standard spectrophotometry with high accuracy assay kits obtained from ZiestChem (Tehran, Iran). Arbutin powder with a purity higher than 98% was procured from Sigma Aldrich (Germany). All chemicals and solvents were of the highest available commercial grade and were purchased from standard commercial suppliers.
3.3. Experimental Protocol
The animals were acclimated to the laboratory environment for a period of 10 days prior to the commencement of the experiment. During this stabilization time, they had access to food and water ad libitum. The acclimated rats were randomly divided into nine groups of seven each and were treated for 28 days as follows:
• Groups I and II were the controls, and they received the vehicle of normal saline (1 mL oral and 0.5 mL intraperitoneally (IP), respectively).
• Group III was given CCl4 (1 mL/kg IP) twice weekly for 28 days.
• Groups IV, VI, and VIII received arbutin (50 mg/kg IP; 75 mg/kg IP; and 250 mg/kg orally, respectively) administered simultaneously with the CCl4 (1 mL/kg IP) twice weekly for 28 dys.
• Groups V, VII, and IX were administrated arbutin (50 mg/kg IP; 75 mg/kg IP; and 250 mg/kg orally, respectively) for 28 days.
In previous studies, the dosage and timing of CCl4 has been demonstrated to successfully and consistently induce hepatotoxicity (15, 16). The animals were anesthetized with chloroform 24 hours after the last injection and then sacrificed. Blood samples were taken from the rats’ axillary artery, and the liver was removed. Serum was separated from the blood by centrifugation for measurement of biochemical factors, thiobarbituric acid-reacting substances (TBARS), and ferric reducing antioxidant power (FRAP). The liver samples and serum were isolated and stored at -80°C for later analysis. The dose and duration of the injections were chosen according to the results of previous studies.
3.4. Total Antioxidant Activity
Total antioxidant activity was determined by FRAP assay. This method is based on the reduction of ferric-tripyridyltriazine [Fe (III)-TPTZ] complex to ferrous-tripyridyltriazine [Fe (II)-TPTZ] in the presence of antioxidants. The FRAP reagent was prepared using 10 mmol/L TPTZ solution in 40 mmol/L HCl plus FeCl3 (20 mmol/L) and an acetate buffer (0.3 mol/L; pH: 3.6) at a 1: 1: 10 ratio, respectively. The freshly prepared FRAP reagent was warmed to 37°C for 5 minutes. The serum sample or standard (50 μL) was then mixed with 1.5 mL of the FRAP reagent and incubated at 37°C for 10 minutes. The absorbance of the colored Fe (II)-TPTZ was measured at 593 nm and compared with a blank. A FeSO4 solution was used as the standard at various concentrations (125, 250, 500, and 1000 μM) (17).
3.5. Evaluation of Serum TBARS
The TBARS assay was used to measure serum lipid peroxidation to estimate the malondialdehyde (MDA) concentration. The working TBARS solution contained 0.375% TBA and 15% trichloroacetic acid (TCA; Sigma Aldrich) in 0.25 N HCl. The TCA/TBA/HCl reagent was freshly prepared, and 2 mL of this solution was mixed with 0.5 mL of the serum or the standard. The solution was then heated for 15 minutes in a boiling water bath. After cooling, the absorbance was measured at 532 nm against a blank that contained all the reagents minus the serum. Different concentrations of MDA (0, 9, 18, and 30 μM) were used as the standards (18).
3.6. Histological Analysis
Small slices of the liver were excised from the anterior part of the left lobe, fixed immediately in a 10% buffered formalin phosphate solution, embedded in paraffin, and sectioned at 5 µm. The serial sections were stained with trichrome for histomorphometric study and hematoxylin-eosin (H&E) for a blind histological assessment that included an evaluation for portal inflammation, hepatocellular necrosis, and hepatocytes hyperplasia. Examination of the surface areas of the hepatocytes was done using a high-resolution (250x) Olympus CKX41 microscope (Olympus; Tokyo) with a photographic facility. At least 100 hepatocytes were measured from each zone of every liver slice. For each, the cytoplasmic and nuclear areas were calculated using Motic software; other parameters, such as the total cell surface and nuclear/cytoplasm ratio, were obtained from the data using the statistical package for the social sciences (SPSS) software.
3.7. Statistical Analysis
The statistical analysis was carried out using SPSS software, version 15.0 (SPSS, Inc., Chicago, IL, US). The difference between groups was determined by a one-way analysis of variance (ANOVA) with post-hoc Tukey testing. A level of P < 0.05 was defined as statistically significant.
4. Results
4.1. Biochemical
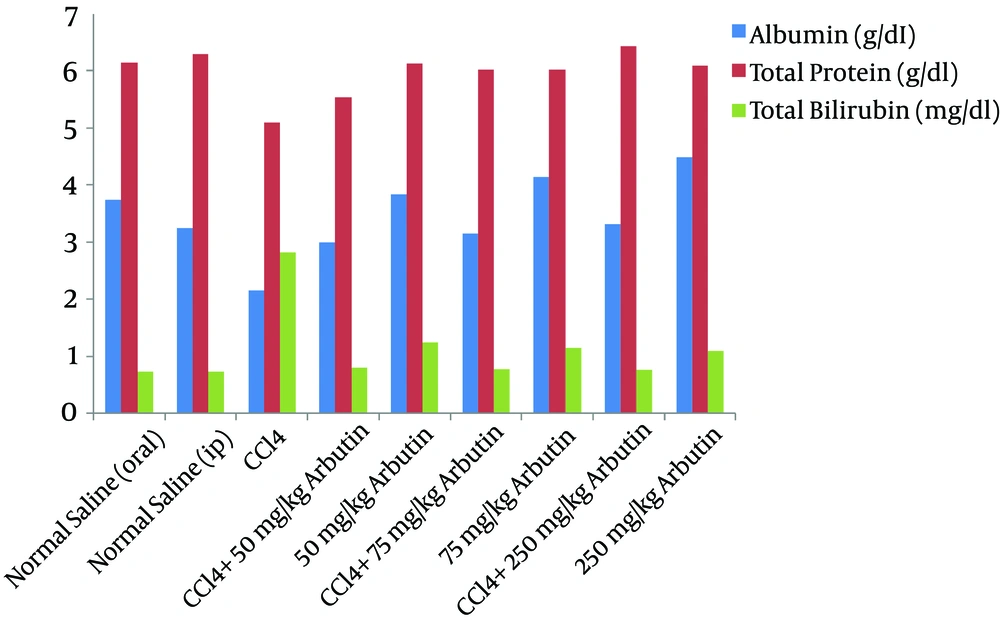
Figure 1 shows the liver function indices for albumin, total protein, and bilirubin concentrations in the serum of rats with CCl4-induced liver damage after the daily administration of arbutin for 28 d. The total protein (TP) decreased significantly for the groups that received CCl4 compared with the normal group (P = 0.001). TP decreased in Groups V, VII, and IX (P < 0.05). The serum albumin concentrations of the groups injected with CCl4 were significantly lower than those of the control groups (P < 0.001), and there was a significant increase in this factor in Groups V, VII, and IX (P < 0.05). Groups IV, VI, and VIII demonstrated a significant increase in the serum bilirubin (P < 0.001). The bilirubin levels in the CCl4-induced groups increased significantly over the normal group (P < 0.001). The bilirubin levels of Groups V, VII, and IX increased but were lower in Groups IV, VI, and VIII (P < 0001).
4.2. Lipid Peroxidation
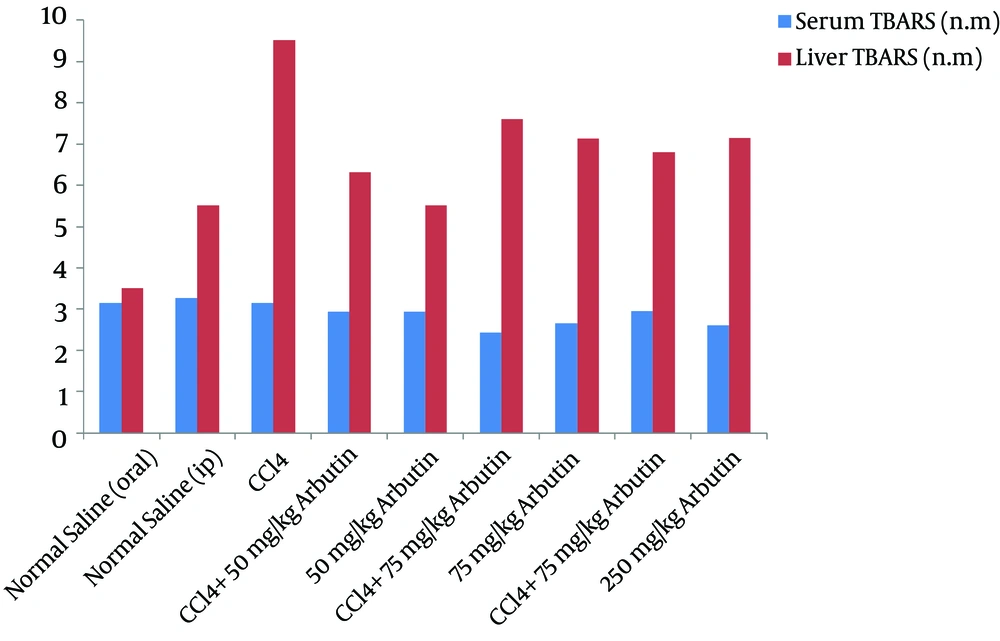
Figure 2 shows that the mean TBARS increased significantly in the CCl4 groups compared with the normal groups (P = 0.001). In Groups IV, VI, and VIII, there was a significant decrease in the mean TBARS (P < 0.05), which was likely due to the decrease in lipid peroxidation in the hepatic tissue. In Group IX, orally administered arbutin showed significant, dose-dependent hepatoprotective activity in comparison with the results for the groups receiving 50 and 75 mg/kg IP injections (Figure 2).
4.3. Antioxidant Capacity
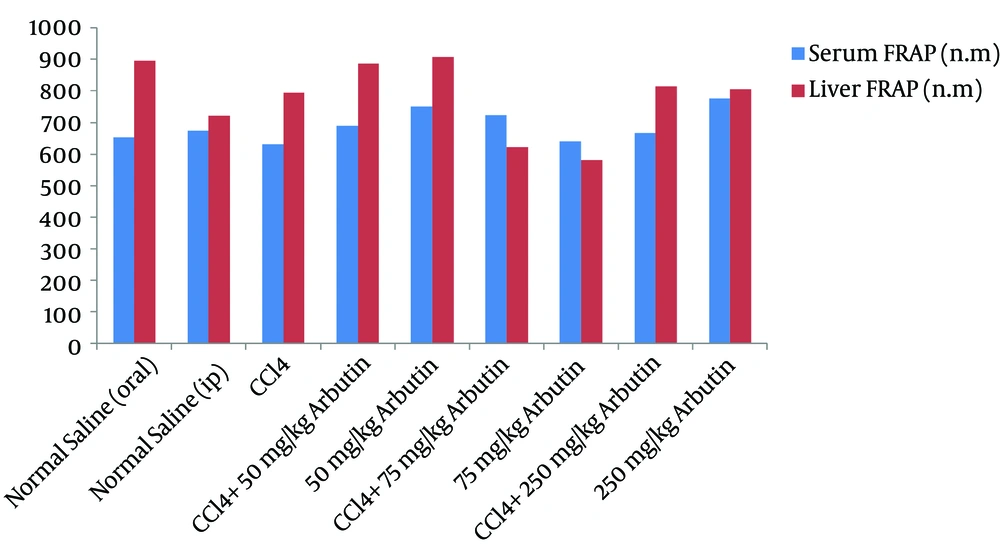
Figure 3 shows that CCl4 injections partially increased liver tissue antioxidant activity (FRAP), but there was no significant difference between the toxin group and the treated groups. A comparison of the groups that received different dosages of arbutin showed a decrease in the antioxidant activity in the liver tissue of the group treated with 75 mg/kg IP of arbutin over the group that was administered 50 mg/kg IP of arbutin (P = 0.017).
4.4. Change in Body Weight
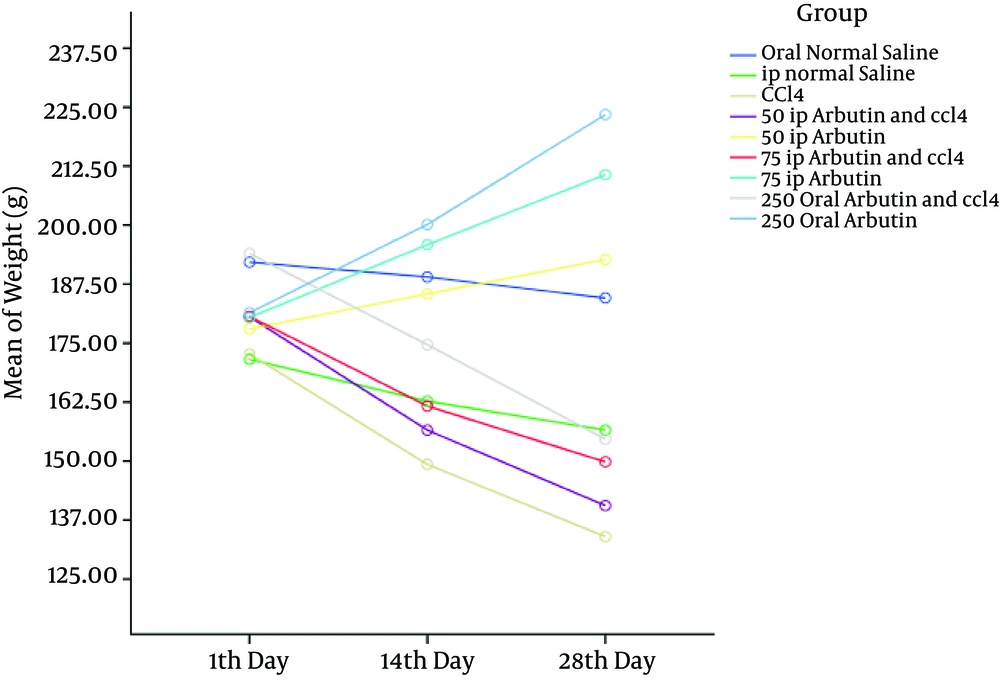
Figure 4 indicates that the body weight of all groups decreased after CCl4 injection but reverted to near normal in the groups that were treated with arbutin (P < 0.001).
4.5. Histological Results
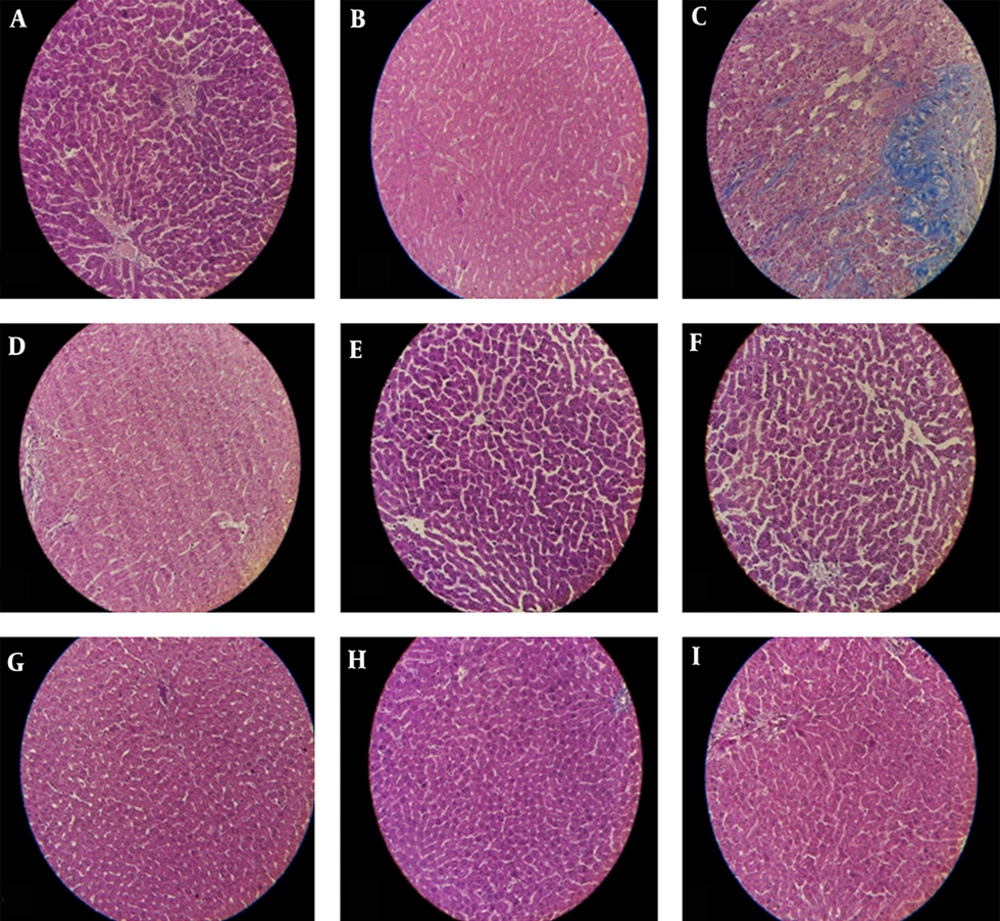
Histopathological studies of the liver in both the control and arbutin-treated animals were normal. Additionally, we observed significant liver destruction, inflammation, fibrosis, and necrosis after CCl4 administration. In rats that received CCl4+arbutin (50 IP), the hepatocyte nucleus was dense, and fibrosis was also noted. In rats that were administered CCl4+arbutin (75 IP), we observed a very similar result that was a little bit milder. Group IX received CCl4+arbutin (250 orally), and its histology was the same as that of the normal groups.
The quantitative parameters of the hepatocyte area, cytoplasmic area, nuclear area, and nuclear/cytoplasmic ratio are given in Table 1 and Figure 5.
| Hepatocyte, µm2 | Cytoplasmic Area, µm2 | Nucleolar Area | Nucleular v/Cytoplasmic Ratio | |
|---|---|---|---|---|
| Oral normal saline | 237.53 ± 1.38 | 143.10 ± 1.35 | 94.43 ± 0.51 | 0.39 ± 0.002 |
| IP normal saline | 241.78 ± 1.68 | 135.48 ± 1.62 | 106.29 ± 0.87 | 0.43 ± 0.009 |
| IP CCL4 | 255.41 ± 2.90b | 149.93 ± 3.83c | 105.47 ± 3.31b | 0.40 ± 0.006b |
| IP CCL4+IP arbutin 50 | 236.31 ± 2.14c | 138.07 ± 2.05d | 98.23 ± 0.77c,e | 0.39 ± 0.003c,e |
| IP Arbutin 50 | 231.40 ± 1.78b,c | 138.02 ± 1.68d | 93.37 ± 0.58c,d | 0.39 ± 0.002c |
| IP CCL4+IP arbutin 75 | 241.00 ± 2.27d | 146.73 ± 2.03b | 94.26 ± 0.85c,d | 0.38 ± 0.003c,e |
| IP arbutin 75 | 235.42 ± 1.91c,d | 142.80 ± 1.84e | 92.62 ± 0.62c,d | 0.38 ± 0.002c |
| IP CCL4+oral arbutin 250 | 253.15 ± 2.58b | 159.21 ± 2.28b,e | 93.94 ± 0.68d | 0.36 ± 0.003c,d |
| Oral arbutin 250 | 239.67 ± 2.34d | 144.48 ± 3.83b | 95.19 ± 3.39b,d | 0.39 ± 0.011b,e |
Quantitative Parameters of the Hepatocyte Area, Cytoplasmic Area, Nuclear Areas, and Nucleolar/Cytoplasmic Ratio in Hepatocytes in the Control, CCl4-Injected, and Arbutin-Treated Groupsa
Typical images were chosen from the different experimental groups (original magnification: X40); (A, B), The control group demonstrated bright and marked nucleoli; (C), The CCl4-injected rats had multiple and extensive areas of portal inflammation and hepatocellular necrosis and a moderate increase in inflammatory cell infiltration and also severe fibrosis; (D), CCl4 and 50 mg/kg IP arbutin: minimal hepatocellular inflammatory cell infiltration and mild portal inflammation and fibrosis; (E), 50 mg/kg IP arbutin was associated with a normal lobular architecture and cell structure; (F), CCl4 and 75 mg/kg IP arbutin produced moderate inflammation and fibrosis; (G), 75 mg/kg IP arbutin was near to normalcy; (H), CCl4 and 250 mg/kg oral arbutin. The liver showed a normal histological profile; (I), 250 mg/kg oral arbutin: the liver showed a normal histological profile.
4.6. Changes in the Total Area of Hepatocytes
The mean hepatocyte area was significantly higher in CCl4-injected rats (P = 0.003) and lower in the arbutin (IP 50 mg/kg) treated group than in that of rats in the normal group. In addition, we saw significant decreases in groups that received CCl4+different doses of arbutin in comparison with the toxic group (P < 0.001).
4.7. Changes in the Nuclear Area
The measurement of the nuclear areas revealed a significant increase in the toxic group (P < 0.001). Furthermore, there was a significant decrease in the groups of different doses of arbutin compared with the CCl4 group (P < 0.001).
4.8. Changes in the Cytoplasmic Area
The mean cytoplasmic area in the toxic group was significantly higher than the level found in normal rats (P < 0.001).
4.9. Changes in the Nuclear/Cytoplasm Ratio
The mean nuclear/cytoplasm ratio in rats treated with arbutin was significantly lower than that of the normal group (P < 0.001).
5. Discussion
The present study showed that the dosage of CCl4 (1 mL/kg) significantly changed the TP, bilirubin, and albumin levels, which is indicative of impaired liver function; these parameters are sensitive indicators of liver damage (19, 20). It is generally accepted that liver damage and hepatotoxicity induced by CCl4 is the best model for screening the protective and antioxidant activity of plant extracts/drugs experimentally (21-23). Numerous studies have indicated that hepatic damage induced by CCl4 is caused by the accumulation of CCl4 in hepatic parenchymal cells that are metabolically activated by cytochrome P-450 dependent monoxygenases to form metabolites of trichloromethyl free radicals (CCl3•) that cause lipid peroxidation and hepatocellular membrane damage. This process is followed by the release of growth factors, inflammatory mediators, and prostaglandins from activated hepatic macrophages that potentiate CCl4-induced hepatic injury (15, 24, 25).
The ability of a hepatoprotective drug to alleviate the injurious effects or conserve normal hepatic physiological mechanisms affected by a hepatotoxin is the index of its protective effects (26). The present study examined the hepatoprotective and antioxidant role of arbutin on CCl4-induced liver damage in rats.
Free radicals affect the pathological manifestation and inflammatory response and can contribute to liver damage (27). The body has an effective mechanism to inhibit and neutralize free radical-induced damage using a set of endogenous antioxidant enzymes. When the balance between ROS production and antioxidant defense is destroyed, oxidative stress results, which deregulates cellular function and leads to various pathological conditions (25). Antioxidants can suppress free radical production, which makes them crucial for protection of the liver from chemical-induced damage through their stabilization of the antioxidant systems in the cell (28).
Prior studies have demonstrated the antioxidative and free radical scavenging properties of arbutin (29, 30). However, no studies thus far have reported on the in vitro cytotoxic activity of this natural compound for normal liver cells to demonstrate its ability to prevent acute tissue damage (12). The low toxicity of arbutin is in accordance with previous in vitro studies on human melanocytes that were exposed to arbutin (31).
One important and sensitive test employed in the diagnosis of hepatic disease is the serum bilirubin concentration, which provides essential information on how well the liver is functioning (32). Bilirubin is a product of the chemical breakdown of hemoglobin that increases water solubility in hepatocytes in conjugation with glucuronic acid (33). The present study showed a significant increase in the bilirubin levels of CCl4-injected rats, which is in accordance with the findings of previous studies (19, 34, 35). This hyper-bilirubinemia may be caused by the inhibition of the conjugation reaction and also the release of unconjugated bilirubin from injured and dead hepatocytes (33).
Serum bilirubin decreased to normal levels after treatment with arbutin, which demonstrates the effectiveness of arbutin in the maintenance of the normal functional status of the liver. Albumin is a key component of serum proteins. It is synthesized in the liver and is one factor used to monitor liver function (36). Studies have shown that liver toxicity decreases serum albumin levels (37). The results of the present study are in agreement with this finding and demonstrate the decreased functional ability of CCl4-injected rat livers (33). Decreased serum TP and albumin levels have been reported in CCl4-administered rats (38). In contrast, a significant increase in the serum TP and albumin levels was observed in the groups that received both arbutin and CCl4. Stabilization of the serum protein levels by treatment with arbutin is a clear indication of an improvement in the functional status of the liver cells.
Increasing evidence supports the hypothesis that CCl4-induced liver damage may be a consequence of oxidative stress (39, 40). The results of this study are in agreement with this hypothesis. Previous studies have demonstrated the antioxidant activities of arbutin (41). Lipid peroxidation has been involved in the pathogenesis of hepatic injury by free radical derivatives of CCl4 and is responsible for cell membrane damage and the subsequent release of marker enzymes of hepatotoxicity (22, 40, 42). In the present study, significantly elevated levels of TBARS, the product of membrane lipid peroxidation observed in CCl4-administered rats, indicated hepatic damage. In contrast, treatment with arbutin prevents lipid peroxidation and may be attributed to its radical scavenging antioxidant constituents (43).
The observed histopathologic changes indicating liver damage after CCl4 administration confirmed the results of the biochemical studies. It was previously reported that CCl4 causes liver necrosis, steatosis, degeneration of hepatocytes (44), and cirrhosis (7, 45). CCl4 was also found to precipitate apoptosis in the liver (46). The histopathologic findings in the liver caused by CCl4 administration in the present study are in agreement with the results of previous studies. Pretreatment with arbutin significantly improved the structure of hepatic cells.
These results suggest that arbutin is effective at reversing the hepatotoxicity caused by CCl4. Morphometric studies have confirmed these observations. The increased cytoplasmic area in the toxic group may be the result of smooth endoplasmic reticulum proliferation in hepatocytes (47, 48). Nuclei are responsible for RNA production and the consequent cytoplasmic protein synthesis (49, 50). The antioxidant effects of arbutin may therefore contribute to the partial or total alleviation of such damage. The histological and biochemical results agree.
5.1. Conclusion
This study showed that arbutin provides protection from the effects of CCl4-induced hepatocellular damage, which is likely due to its ability to scavenge free radicals. Liver cells treated with arbutin suppressed the changes that were induced by CCl4. These findings suggest that arbutin can be used as an antioxidant to protect the liver from the oxidative damage induced by toxic chemicals. More studies are needed to examine the exact mechanisms operating in arbutin and to evaluate its therapeutic effect on hepatic injury induced by chemicals.