1. Background
Lippia citriodora belongs to the Verbenaceae family and includes 200 species of herbs, shrubs, and small trees (1, 2). Most plants of this family have traditionally been used as remedies for gastrointestinal and respiratory problems. L. citriodora is mainly distributed in South America, North Africa, Southern Europe, and the northern region of Iran (1, 3) and has a long history as a folk remedy for the common cold, asthma, colic, fever, diarrhea, indigestion, insomnia, and anxiety (4-6). It is cultivated generally for the lemon-like aroma emitted from its leaves, which are used to prepare herbal tea (7), most commonly as a decoction or infusion that is administered orally. It is believed that the essential oil and phenolic compounds of L. citriodora are responsible for pharmacological properties including anti-inflammatory, antimicrobial, and antitumor activity. L. citriodora extract also contains several phenolic compounds, such as phenylpropanoids and glycosilated flavones (8-10). Phenylpropanoids are the most widespread compounds in lemon verbena extract. Verbascoside, the most abundant phenylpropanoid glycoside from lemon verbena, is reported to possess many biological features, including anti-oxidative, antibacterial, anti-tumor, and antifungal actions (11-13).
2. Objectives
The increasing use of L. citriodora and lack of scientific data on its safety profile make it necessary to evaluate its safety. In the present study, an effort was made to evaluate the acute, subacute, and cellular toxicity of the aqueous extract of L. citriodora leaves.
3. Methods
3.1. Plant Material and Aqueous Extract Preparation
L. citriodora leaves were collected from the Karaj region of Iran, dried in shadow, and ground to a smooth powder. L. citriodora was properly identified by the Department of Botany of Ferdowsi University, and voucher samples were preserved for reference in the herbarium of the Department of Pharmacognosy, School of Pharmacy, Mashhad, Iran (1203-1).
For aqueous extract preparation, one liter of distilled water was added to 100g of dried leaf powder and boiled for 20 minutes. The mixture was filtered through Whatman No. 1 filter paper and then concentrated by rotary evaporator. The obtained residues were kept in a freezer at -55°C until use.
3.2. Animals
Male BALB/c mice (25 - 30 g) were selected for the acute toxicity studies, and male Wistar rats (200 - 250 g) were selected for the subacute toxicity studies. They were obtained from the animal house of the pharmacy school of Mashhad University of Medical Sciences and maintained under a controlled environment, including a 12-hour light/dark cycle at 21 ± 2°C, with free access to water and food. All animal experiments were approved by the Animal Care Committee of the Mashhad University of Medical Sciences.
3.3. Acute Toxicity
Acute toxicity studies were carried out in 24 animals using four different doses (14). Three groups were treated with different doses of aqueous extract (1, 2, and 5 g/kg of body weight) via intraperitoneal (IP) injections, and the control group received the same volume of normal saline (0.25 mL/25 g of body weight). After single dose administration, the animals were observed for general behavioral changes, signs of toxicity, and mortality (15).
3.4. Subacute Toxicity
In this experiment, four groups of six rats each received 0, 50, 100, and 200 mg/kg of extract via IP for 21 days. The control group received the same volume of normal saline (0.5 mL/250 g of body weight). The animals were observed for mortality and clinical signs until the end of the experiment. The body weight was recorded weekly. Food and water intake was recorded every day. On the 21st day, the animals were anaesthetized by chloroform, and blood samples were collected for hematological and biochemical analysis (16).
3.5. Hematological and Biochemical Parameters
At the end of the treatment period, the animals were sacrificed under chloroform anesthesia, and blood samples were obtained by cardiac puncture into heparinized and non-heparinized tubes. Heparinized samples were used immediately for the analysis of hematological parameters, such as red blood cell count, hemoglobin, platelet count, and white blood cell count by automated hematology analyzer. For the biochemical parameter analysis, the levels of AST, ALT, LDH, total bilirubin, total cholesterol, triglyceride, serum glucose, albumin, urea, and creatinine were determined.
3.6. Histopathology
After blood collection, vital organs, such as the liver, kidney, lung, brain, heart, and spleen, were removed and then fixed by immersion in 10% formalin. Following fixation, the tissues were dehydrated in an ascending series of ethanol, embedded in paraffin, and then sectioned at 6-µm thickness. Tissue sections were stained with hematoxylin and eosin for histopathological study.
3.7. Cell Viability Assay
The in vitro cytotoxicity was studied in HepG2 cells (National Cell Bank, Pasteur Institute, Tehran, Iran) using the 3-(4, 5-dimethyl thiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay. Harvested cells were seeded into a 96-well plate with different amounts of aqueous extract (0, 50, 100, 200 µg/mL) for 24 and 72 hours. After treatment, MTT solution was added to each well and incubated for 1 hour at 37°C. Subsequently, the insoluble formazan crystals were dissolved in dimethyl sulfoxide (DMSO) and the absorbance was measured at 570 nm in plate readers (Bio-Tek, ELX 800, USA) (17, 18).
3.8. Statistical Analysis
Data was expressed as mean ± SEM. All data was analyzed using analysis of variance (ANOVA), followed by Tukey-Kramer. Statistical significance was considered as P values less than 0.05 (P < 0.05).
4. Results
4.1. Acute Toxicity
A single IP injection of L. citriodora extract at 2 and 5 g/kg produced 1 and 3 deaths per group within 24 hours of treatment, respectively. Thus, the LD50 value was calculated as 5 g/kg of body weight. Normal saline injection did not cause any death or toxicity in mice.
4.2. Subacute Toxicity
No symptoms of toxicity or behavioral changes were recorded during the course of the study. Daily IP injection of L. citriodora extract at different doses had no effect on the mean body weights of rats (data not shown), compared with the control group. No difference in food or water consumption was observed among the groups.
4.3. Hematological and Biochemical Parameters
In the present study, no significant changes were observed in hematological parameters including hemoglobin, hematocrit, platelet count, white blood cell count, and red blood cell count of the aqueous extract-treated groups in comparison to the control group (Table 1). Similarly, none of the biochemical parameters, such as creatinine, amylase, lipase, CPK, LDH, urea, total cholesterol, total protein, albumin, alkaline phosphatase (ALP), and total bilirubin of the experimental treated groups displayed significant changes when compared to control. However, the aqueous extract, at doses of 50 and 100mg/kg, decreased triglyceride levels (P < 0.01) (Table 2).
| Parameters | Control | Extract (50 mg/kg/day) | Extract (100 mg/kg/day) | Extract (200 mg/kg/day) |
|---|---|---|---|---|
| WBC (× 103/μL ) | 9.43 ± 1.3 | 11.1 ± 1.1 | 8.33 ± 0.9 | 9.38 ± 0.9 |
| RBC (× 106/μL) | 6.85 ± 0.2 | 6.70 ± 0.1 | 6.58 ± 0.2 | 6.66 ± 0.1 |
| Hemoglobin (g/dL) | 12.8 ± 0.59 | 11.71 ± 0.28 | 11.51 ± 0.32 | 12.48 ± 0.70 |
| Hematocrit (%) | 38.13 ± 1.5 | 35.56 ± 0.7 | 34.96 ± 0.6 | 36.51 ± 1.3 |
| Platelets (× 103/μL) | 553.05 ± 75.2 | 569.83 ± 46.02 | 550 ± 44.2 | 532.2 ± 6.8 |
Abbreviations: WBC, white blood cells; RBC, red blood cells.
aValues are expressed as mean±SEM (n = 6).
| Parameters | Control | Extract (50 mg/kg/day) | Extract (100 mg/kg/day) | Extract (200 mg/kg/day) |
|---|---|---|---|---|
| Amylase (IU/l) | 31.83 ± 3.27 | 34.83 ± 2.93 | 40.83 ± 2.54 | 40.66 ± 2.36 |
| Lipase (IU/l) | 34 ± 2.129 | 37.8 ± 3.36 | 40.5 ± 4.10 | 44 ± 2.92 |
| CPK (IU/l) | 12593.3 ± 404.9 | 12714 ± 1497 | 11426 ± 1439 | 14522.16 ± 1190.4 |
| LDH (IU/L) | 1513 ± 13.04 | 1859 ± 10.20 | 1962 ± 16.69 | 1929 ± 14.19 |
| Tg (mg/dL) | 102 ± 3.66 | 53 ± 2.8b | 49 ± 2.58b | 50 ± 4.0 9b |
| Cholesterol (mg/dL) | 73 ± 3.85 | 66.5 ± 4.44 | 59 ± 3.0 | 61 ± 3.9 |
| Protein (g/dL) | 6.2 ± 0.198 | 5.7 ± 0.071 | 5.63 ± 0.138 | 5.7 ± 0.215 |
| Creatinine (mg/dL) | 0.916 ± 0.0307 | 0.983 ± 0.0749 | 1.08 ± 0.0542 | 0.93 ± 0.0614 |
| T. Bilirubin (mg/dL) | 1.66 ± 0.3127 | 0.75 ± 0.145 | 0.98 ± 0.166 | 1.45 ± 0.355 |
| Urea (mg/dL) | 30 ± 2.6 | 29 ± 4.5 | 34.5 ± 2.1 | 25 ± 3.3 |
| Albumin (g/dL) | 3.7 ± 0.101 | 3.65 ± 0.056 | 3.45 ± 0.061 | 3.6 ± 0.162 |
| ALP IU/L | 2.866 ± 0.035 | 2.832 ± 0.032 | 2.906 ± 0.084 | 3.601 ± 0.081 |
Abbreviations: ALP, alkaline phosphatase; LDH, lactic acid dehydrogenase; Tg, triglyceride.
aValues are expressed as mean ± SEM (n = 6).
bP < 0.001 vs. control.
4.4. Histopathological Study
The microscopic examination of internal organs did not indicate any histological changes when compared with the control group. Only one control showed congestion in heart specimens.
4.5. Cell Viability
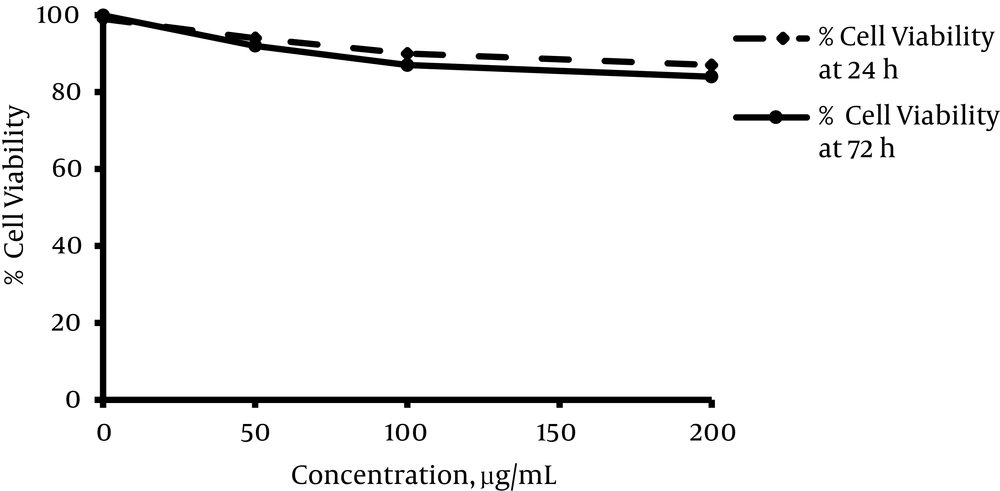
The viability of HepG2 cells was evaluated after 24 and 72 hours’ exposure to different concentrations of L. citriodora aqueous extract. As shown in Figure 1, the maximum concentration of extract (200 µg/mL) did not reach 50% of cell death. Overall, the cytotoxicity assay revealed that viability in all groups was greater than the IC50 value (17).
5. Discussion
Although use of plants as natural sources of medicinal remedies has expanded sharply in recent decades, this use does not mean all plants are safe or free of side effects. Various studies have noted the potential risks involved with such plants (19). L. citriodora is one medicinal herb with a broad spectrum of therapeutic potential that needs to be evaluated for possible toxic effects (20).
The results of our study showed the intraperitoneal LD50 value for mice is 5mg/kg of body weight, which can be considered safe. The evaluation of the acute toxicity of L. citriodora cultivated in Egypt (L. citriodora Kunth) confirmed our results and revealed that aqueous extract had an LD50 grated than 5 g/kg (6.4 g/kg of body weight) (21).
Similarly, body weight and food and water consumption during the 21-day experiment were not affected, nor did the experiment produce any behavioral symptoms. In contrast, Pastorelli et al. found that giving feed supplemented with a Verbascoside-rich diet (10 mg/kg) for 56 days significantly improved the growth performance of pigs. During the entire experimental period, the average daily gain, the gain/feed ratio, and final weight increased in the group that received the highest dose (11). Our results did not confirm these results, which may be due to the short duration of feeding.
Our data concluded that the hematological parameters of the treatment and control groups were within normal range. Subacute IP administration of L. citriodora extract also did not show any significant changes in biochemical parameters, except a decrease in the level of Tg. No significant difference in ALT content, normal serum level of cholesterol, or LDL in the control and extract-treated groups was revealed, and there was an absence of any harmful effects on the liver. However, Verbascoside can significantly decrease levels of Tg, and therefore may be useful in the treatment of hypertriglyceridemia. Similarly, no significant changes in other biochemical parameters, such as creatinine and BUN, indicate that there is no adverse effect on kidney function after 21 days’ IP injection of L. citriodora leaf extract at different doses. Plasma protein and albumin measurement may act as an indicator of the synthetic capacity of the liver (22, 23). Therefore, no change in these factors suggests an absence of abnormality in the synthetic capacity of the liver. In one clinical trial, a large number of participants was randomly divided into three groups that received daily administration of olive oil with low, medium, or high phenolic contents. All interventions increased the level of HDL cholesterol and decreased the total cholesterol/HDL ratio, cholesterol, and triglyceride levels. Due to these findings, Covas et al. proposed polyphenol-rich olive oil as a good source of fat to achieve additional benefits against cardiovascular risk factors (24). In a study conducted by Pastorelli et al., as mentioned above, serum low-density lipoprotein cholesterol was reduced by 17% in the high Verbascoside group at day 56. No change in other biochemical parameters, such as glucose, urea, total cholesterol, triglycerides, or high-density lipoprotein cholesterol serum concentrations, was reported in this study (11). It is concluded that phenolic compounds, especially Verbascoside, in large amounts, have a beneficial effect on the biochemical profile and, since an aqueous solvent extracts less phenolic compounds, it may have fewer pharmacological effects (15, 25).
A similar absence of toxic effects was observed in the cell viability assay. Pesce et al. recently demonstrated that Verbascoside, at concentrations from 10 to 250 μm, does not affect the viability of U937 mononuclear cells, though it decreases the cell viability at a concentration of 500 μm by at least 50%. In accordance with our results, verbascoside showed relatively low cytotoxic effects on HepG2 and U937 cell lines (26). Moreover, histopathological examination of all organs indicated no abnormal findings. In our previous work, L. citriodora extract administered for 10 days to pregnant mice also showed no teratogenic effects (27).
Results from the present study elucidate that treatment with the aqueous extract of L. citriodora leaves was well tolerated in daily IP injection at doses up to 200 mg/kg for a period of 21 days and did not produce any toxicity.