1. Background
Diabetes is a chronic metabolic abnormality consisting of severe and chronic hyperglycemia, resulting from impaired both insulin function and secretion and its prevalence have reached an epidemic worldwide (1). Diabetic nephropathy (DN) is one of the most leading causes of renal failure which increases the mortality of diabetic patients. The precise mechanisms causing the development and progression of renal damage are not yet completely understood (2). Recently, several pathways involving the progression of diabetic nephropathy have been determined and given evidence emphasized for the significant role of inflammation in the development of diabetic-induced kidney disease (3). Nuclear factor kappa B (NF-κB), generally present a p50/p65 heterodimer, is a key multifunctional transcription factor, which involves in the inflammation (4). NF-κB generally expresses in all cells and its inactive form commonly present is bound to an inhibitory subunit (I-κB) in the cytoplasm (4). It is expected that NF-κB has a vital role in the diabetic nephropathy, and increasing data shows that it has an important role in the structural and functional changes, which occurred in the DN (5). In general, a wide variety of activators could stimulate the NF-κB signaling pathway, however, in diabetic kidney disease, proteinuria and angiotensin II are both important activators for stimulation of NF-κB (6, 7). Hyperglycemia, a major risk factor of diabetic nephropathy, could stimulate non-enzymatic protein glycation, the formation of reactive oxygen species (ROS), and activation of protein kinase C (PKC) (8, 9). All of these pathways increase inflammatory cascade that promotes fibrosis and renal damage. Therefore, suppression of NF-κB activation by using anti-inflammatory agents may suggest as a therapeutic target for diabetic nephropathy (10).
Ginger (Zingiber officinale Rosces) is a flowering plant whose rhizome, roots, or simply ginger, has anti-inflammatory, anti-oxidant, anti-hypertensive, and hypoglycemic properties (11). Ginger roots and its constituent showed a key role in the treatment of diabetes and its complications via anti-hyperglycemic effects (12). It has been suggested that the mechanism action of ginger in the treatment of diabetic complications may be mediated by inhibition of oxidative stress and its anti-inflammatory effect (13). Moreover, it has been demonstrated that ginger can prevent the activity of the NF-κB signaling pathway (14). Previous investigations revealed that Zerumbone, a tropical ginger sesquiterpene, could ameliorate the progression of structural kidney damage in diabetic rats (15). The possibility of protective effects of the ginger hydroalcoholic extract on the increased renal P-p65 level and thereby attenuated the histopathological abnormalities of diabetic nephropathy were assessed in the present study.
2. Methods
2.1. Animals and Sample Collection
Sixteen male Wistar rats weighing 200 - 250 g (eight to ten weeks of age) were obtained from the Research Center and Experimental Animal House of Ahvaz Jundishapur University of Medical Sciences. The current study was performed based on the approval of the Ethical Committee of Animal Breeding and Research of Ahvaz Jundishapur University of Medical Sciences (approval number: HLRC-9403). Rats were kept in a temperature-controlled room with a 12-hour light-dark cycle and received a standard diet and water. For induction of diabetes, the rats fasted overnight and subjected for a single intraperitoneal injection of 60 mg/kg STZ (Sigma-Aldrich, USA) solution in citrate buffer. Fasted rat with blood glucose level ≥ 350 mg/dL were considered as diabetic. The animals were divided into four experimental groups and treated for eight weeks as follows.
• Non-diabetic control: The healthy rats receive 1.5 mL/kg distilled water orally once daily.
• Diabetic control: The diabetic rats receive 1.5 mL/kg distilled water orally once daily.
• Diabetic treated: The diabetic rats receive 200 mg/kg of the extract orally once daily.
• Diabetic treated: The diabetic rats receive 400 mg/kg of the extract orally once daily.
At the end of treatment, the rats were weighted, and urine samples were collected for 24 hours in individual metabolic cages. Blood samples were collected directly from the animal’s heart by using not heparinized syringes. Both kidneys were dissected from each rat and weighting was performed after being washed in cold isotonic solution. One kidney was kept at -80°C in the liquid nitrogen condition for western blotting. In addition, the other kidney for histological studies was fixed in 10% formalin. Serum was separated and kept at -80°C for measurement of biochemical variables. The concentration of blood glucose and serum creatinine level were determined by the biochemical analyzer
2.2. Preparation of Z. officinale Extract
First of all, the rhizome of Z. officinale was obtained from a regional corporation, Gol Darou, Isfahan, Iran. Then, 200 gr of the rhizome was beached by an electric mixer, and drenched with methanol solution (70%) during 72 hours. Finally, after filtration by a Whatman filter paper, the obtained extract was concentrated in an evaporator and dried by a Freeze Dryer. The yield of obtained dried powder was 12.5 %.
2.3. Western Blotting
Western blot analysis was carried out on the crude lysate, which was obtained from homogenized kidney cortex in lysis buffer containing 1% Triton X-100, and a cocktail of protease inhibitor (Roche, Mannheim, Germany). The homogenate was centrifuged (16000 g, 20 minutes at 4°C) and the supernatant protein concentration was assessed with Bradford assay. For electrophoresis 50 μg of protein was loaded on the polyacrylamide gel containing 10% sodium dodecyl sulfate (SDS-PAGE). The blots were then transferred to PVDF membrane and blocked with bovine serum albumin (BSA), 5% in phosphate-buffered saline (PBS), for one hour. Following, the blots were incubated with a specific antibody against p-NF-κB p65 (ser 536, sc-20150, 1:2000 dilution), and re-incubated for β-actin (sc-130656) as a loading control. The membranes were washed three times for 10 min in TBS/T buffer and then incubated in goat anti-rabbit peroxidase-conjugated antibody (sc-2030, 1/2000 dilution) in TBS/T buffer for one hour at room temperature. Lastly, the protein blots were detected by Bio-Rad ChemiDocTM (Hercules, CA, USA) and densitometry performed using ImageJ software.
2.4. Kidney Histological Analysis
For the histological studies, first, the kidneys were fixed in formalin (10%), and then for dehydration recovered with an increasing percentage of alcohol content, and were embedded in paraffin. The 5µm-thick sections of kidneys were stained with periodic acid-Schiff (PAS) and were evaluated by light microscope.
2.5. Statistical Analysis
In all statistical analysis, the data are expressed as mean ± standard deviation (SD). The data were analyzed with one-way analysis of variance (ANOVA) test, and the Tukey post-hoc test was used to determine the source of significant differences between groups. Statistical significance was accepted at the level of P < 0.05.
3. Results
3.1. Effects of Z. officinale Extract on Blood Glucose and Body Weight
At the end of the study, blood glucose concentration in diabetic rats was significantly increased compared to non-diabetic control (P < 0.01). Whereas, after treatment with the extract a significant reduction of blood glucose concentration was observed (P < 0.01) (Table 1). Table 1 shows that the mean body weight in diabetic rats significantly decreased (P < 0.01), however, after treatment with the extract body weight increased.
Abbreviations: BW, body weight; CO, healthy control; DM, diabetic control; DM+200, diabetic treated with 200 mg/kg of the extract; DM+400, diabetic treated with 400 mg/kg of the extract; FBS, fasting blood glucose, KW, kidney weight.
aValues are expressed as mean ± SD.
bComparisons were made using one-way ANOVA followed by Tukey’s post hoc test.
cSignificant difference with CO.
dSignificant difference with DM.
3.2. Effects of Z. officinale Extract on the Kidney Function Indices
In diabetic control, an increase in 24-hour urine volume, accompanied by an increase in urine protein excretion was observed. However, both of them were reduced in diabetic rats treated with the extract (P < 0.01). In addition, serum creatinine concentration in untreated diabetic control was markedly increased (P < 0.01). Moreover, a significant reduction of creatinine in diabetic rats treated with 400 mg kg-1 of the extract was detected (P < 0.05) (Table 2).
Abbreviations: CO, healthy control; DM, diabetic control; DM+200, diabetic treated with 200 mg/kg of the extract; DM+400, diabetic treated with 400 mg/kg of the extract.
aValues are expressed as mean ± SD.
bSignificant difference with CO.
cSignificant difference with DM.
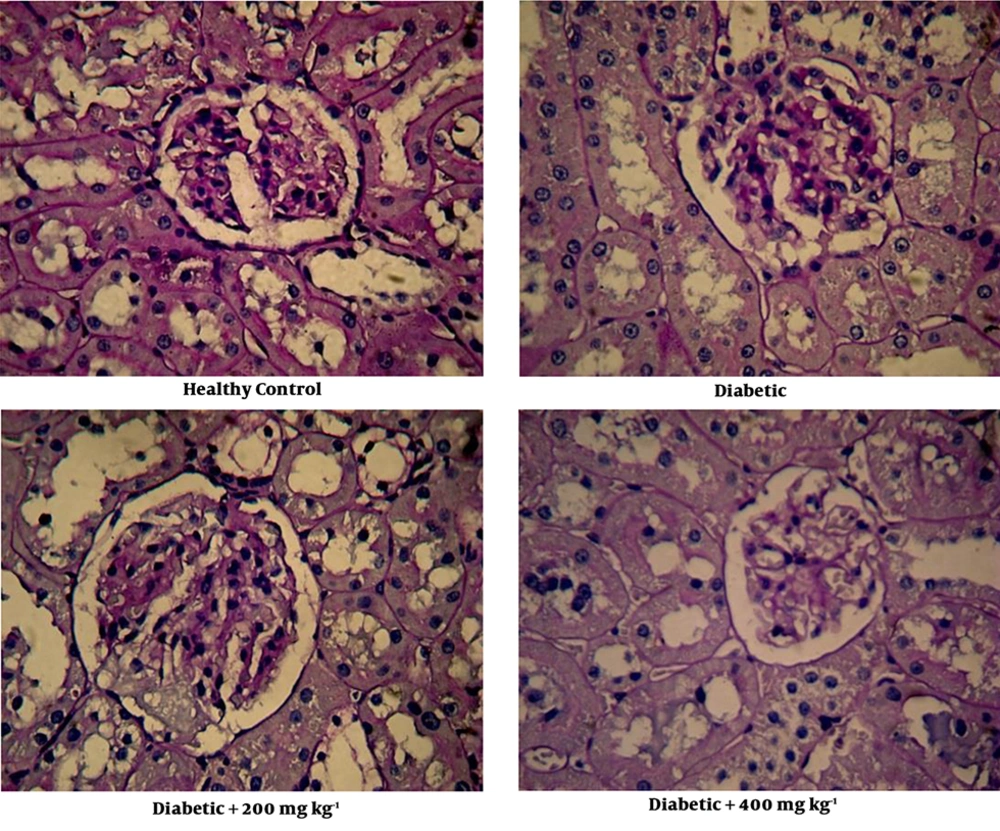
3.3. Effects of Z. officinale Extract on the Kidney Morphology
At the end of the intervention, the mean of kidney weight and the kidney weight to body weight ratio in diabetic control were significantly increased compared to non-diabetic control. However, in diabetic rats treated with 200 mg/kg/day of the extract a significant reduction of kidney weight to body weight ratio was detected compared to diabetic control. In addition, in diabetic rats treated with 400 mg/kg/day of the extract an obvious reduction in kidney weight and kidney weight to body weight ratio was detected (Table 1).
3.4. Effects of Z. officinale Extract on the Kidney Histology
In diabetic control, a focal mesangial matrix expansion and renal corpuscle with a bit hypertrophy were observed (Figure 1). After treatment with 200 mg/kg of the extract, expansion of the mesangial was slightly attenuated (Figure 1). Treatment with 400 mg/kg of the extract ameliorated enlargement of the mesangial in glomeruli. Figure 1 also shows renal corpuscle size was reduced in diabetic rats treated with the extract.
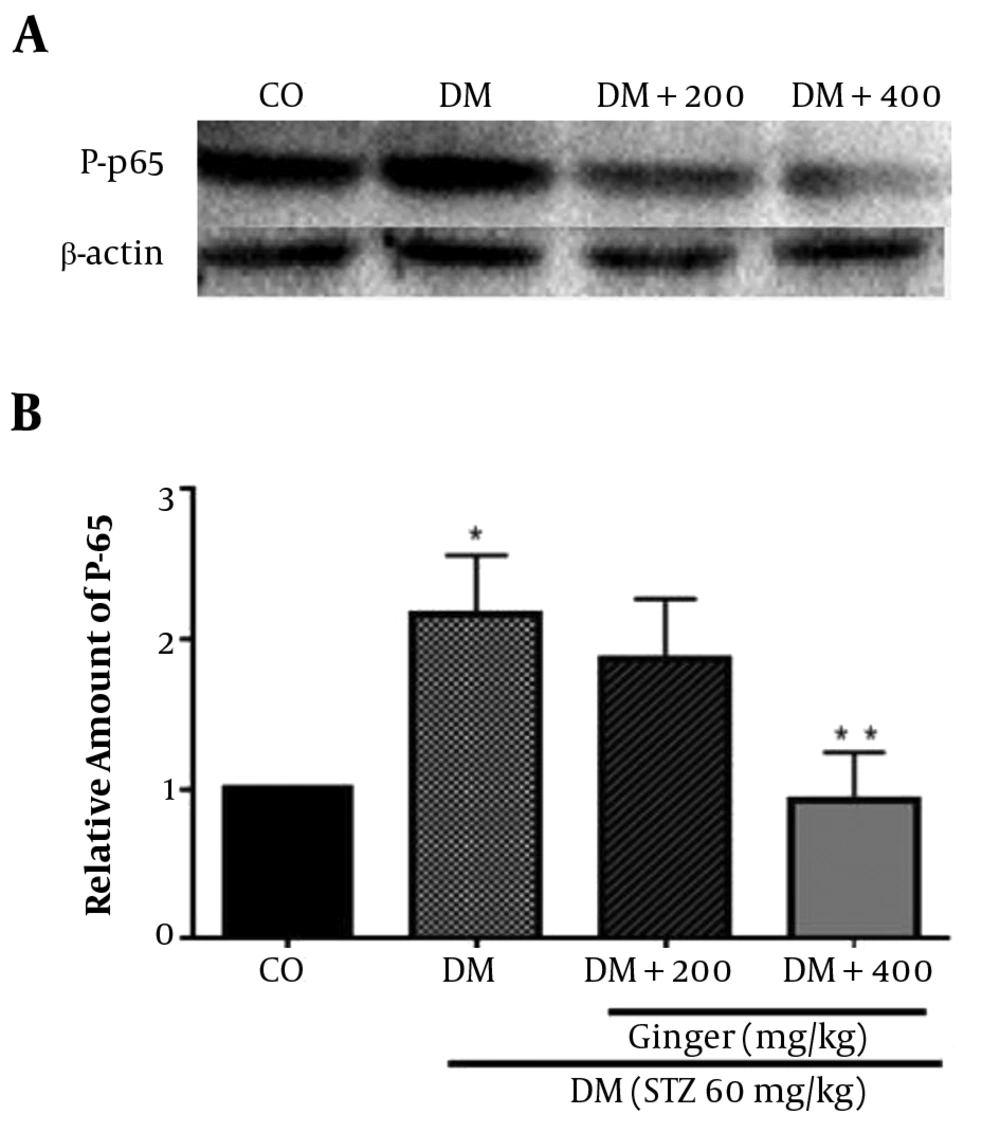
3.5. Effects of Z. officinale Extract on P-p65 Protein Level
The immunoblotting analysis showed that the level of phosphorylated p65 protein was higher in kidney tissues of diabetic control (P = 0.02). After oral administration of the extract at 400 mg kg-1, P-p65 protein level was reduced (P = 0.04). However, after treatment with 200 mg kg-1 of the extract, no significant reduction in the level of P-p65 was detected (P = 0.2) (Figure 2).
Effects of Z. officinale extract on the P-p65 protein level (ser536). A: At the end of intervention, the rat’s kidneys from each group were collected and homogenized to detect p-p65 level by Western blot analysis. β-actin was used as loading control. B: Ratios of p-p65/β-actin level are expressed as mean ± SD (n = 4 per group) in each column. *, significant difference with healthy control; **, significant difference with diabetic control. CO, healthy control; DM, diabetic control; DM+200, diabetic treated with 200 mg/kg of the extract; DM+400, diabetic treated with 400 mg/kg of the extract.
4. Discussion
Among various methods for extraction of interest constituent from plants, an efficient extraction method is that it could extract with the higher product, and with least alterations in the bioactive component of the product. Therefore, the purpose of all extractions is to separate the soluble plant component, leave out the insoluble cellular residue. If the extraction is performed with a mixture of ethanol, or methanol with water according to their polarities, they contain all component of the plant which called total extract. Recently, most of the extracts used in medication as oral drugs are hydroalcoholic extract (16). According to these explanations we used the hydroalcoholic extract of ginger in the current study. Lately, the safety of ginger supplement has been increased and a toxicological study conducted in rats showed a wide-ranging of safety for ginger supplementation. For oral administration in rats, the severe cytotoxicity (LD50) dosage of the alcoholic and aqueous ginger extract was 10.25 g/kg and 11.75 g/kg, respectively (17). According to the previous report, in the current study 200 mg/kg and 400 mg/kg of the ginger extract was used for intervention (18).
Our results indicated a significant reduction in plasma glucose concentration in diabetic rats treated with the extract. An important finding in STZ-induced type 1 diabetic rat showed an ethanolic extract of ginger (800 mg/kg) when orally administrated could significantly decrease the blood glucose concentration (13). Also, in a study conducted in diabetic and non-diabetic animals results showed that ginger juice has a significant lowering effect on the blood glucose concentration (19). Results from several studies conducted in type 1 diabetic animals found that long-time treatment with ginger could decrease blood glucose, serum triglyceride (TG), and total cholesterol (TC), and increase serum insulin, and kidney weight loss (20-22). A study on high-fat-diet-induced diabetes, indicated that treatment with an ethanolic extract of ginger, can reduce the increased body weight, serum glucose, insulin, TC, LDL-C, TG, FFA, and phospholipid (18). Moreover, the major spicy component of ginger, 6-gingerol, has a significant reduction in blood glucose level and recovered glucose tolerance in type 2 diabetic mice and lower serum TG, TC, FFA, LDL-C, and insulin levels (23). Moreover, in insulin-sensitive peripheral tissues, ginger could increase glucose uptake, which is a key step for blood glucose regulation. Several in vitro studies showed ginger extract and its spicy constituent, gingerol, could increase the uptake of glucose in rat skeletal muscle cells and adipocytes (24-26). In hyperglycemic condition, the formation of advanced glycated end products (AGE) is increased, which will play an important role in diabetic nephropathy. A study conducted in diabetic rats, showed that after oral administration of ginger the formation of several AGE products, was significantly reduced (27).
Clinically, DN is characterized by macroalbuminuria where urinary albumin excretion is more than 300 mg/24 h, and abnormality in renal function presented by an increase in serum creatinine concentration (28). Our results indicated a significant reduction in the amount of urine proteins accompanied by reduction of urine volume in diabetic rats treated with the extract. Also, serum creatinine significantly increased in diabetic controls, while, after treatment with 400 mg/kg of the extract a significant reduction of serum creatinine was observed. Tzeng et al., reported that in STZ-induced diabetic rats, treatment with zerumbone, tropical ginger, proteinuria, and urine volume was decreased due to its effect on the reduction of blood glucose (15). Moreover, Eleazu et al., reported the reduction of proteinuria in rats treated with ginger causes an improvement of glomerular complications (29).
In the current study, we observed the body weight in diabetic control significantly reduced. It is speculated that high blood glucose induces weight loss in diabetic rats via loss of valuable fluids and body water. In addition, loss of body weight may be mediated by the progressive destruction of muscular proteins (30). Our results indicated treatment with the extract protected the rats against diabetic-induced body weight loss, which may be mediated by the potential beneficial effect of ginger in the reduction of blood glucose. Moreover, kidney weight in rats treated with the extract significantly increased. Suggested, an increase in kidney weight may be result from the beneficial effect of ginger extract on the regeneration and repair of kidney cells (11). Tzeng and Eleazu reported an increase in kidney weight in diabetic rats treated with ginger may be due to the restoration of kidney’s cells after reduction of proteinuria (15, 29).
It has been reported that high blood glucose can increase the activity of NF-κB through induction of IκBα (inhibitor of kappa B) phosphorylation in the cultured of human HUVEC cells (30). In addition, Grzanna et al., reported the destruction of the IκB and translocation of P-65 into the nucleus occurred in a culture of kidney epithelial cells and diabetic mice in response to increased glucose concentration (31). In our study, the P-p65 protein level, an active subunit of the NF-κB, was increased in diabetic control, however, in diabetic treated with 400 mg/kg of the extract was significantly decreased. In a study, Tzeng et al., reported Zerumbone inhibits TNF (Tumor Necrosis Factor)-induced NF-κB activity in rats. Also, inhibition of NF-κB by Zerumbone is associated with the suppression of IκB kinase activity, by phosphorylation of IκB, and destruction of IκB, phosphorylation, and acetylation of P65 (28). The previous study reported that ginger and some related compounds have anti-inflammatory effects such as inhibition of cyclooxygenase and inhibition of the NF-κB pathway (29). Therefore, the effect of ginger in the reduction of P-p65 protein level may be result from the anti-inflammatory, antioxidant and anti-hyperglycemic properties of this plant. Our findings indicated after reduction in P-p65 protein level, the expansion of mesangium, as well as the size of renal corpuscle in diabetic rats treated with 400 mg/kg of the extract was significantly reduced. Al-Qattan et al., in a study, found that oral administration of ginger extract in diabetic rats could reduce the development and progression of structural changes in kidneys. Also, after treatment with the extract the improvement in the form of renal corpuscle, the integrity of the capsular wall and glomerular arrangement were observed. More important, the reduction of glomerular mesangium was observed in the area of the renal corpuscle (32).
4.1. Conclusions
Our results indicated that the beneficial effect of Z. officinale hydroalcoholic extract in rats with diabetic nephropathy at least mediated by its anti-hyperglycemic effect that accompanied by inhibition of NF-KB signaling pathway through reduction of P-p65 protein level. Further studies are being undertaken to clarify completely the mechanism(s) of the P-P65 protein lowering the effect of Z. officinale.