1. Background
According to evidence of ancient time, early man used herbs as medicine to treat diseases. With the advancement of science, application of chemical compounds drew the attention of researchers to develop medicine, but it did not last long; side effects and inefficiency of such agents made scientists focus on herbal compounds to treat diseases (1). Fighting against infectious diseases is the most important treatment challenge due to their high prevalence. Misuse of antimicrobial drugs led to increased resistance against different antibiotics in most bacteria (2). With increased antimicrobial resistance in most bacteria, researchers performed more studies to develop new antimicrobial agents. Herbs are the best sources to achieve this goal due to being natural, causing lower complications, easy access, and being economical compared to synthetic agents (3, 4). Foeniculum vulgare Mill. (F. vulgare Mill.) is a perennial flavored herb belonging to the family Umbelliferaceae (5). The plant has oval or spindle-shaped seeds and studies indicated antimicrobial activity of fennel and spices containing fennel (6, 7). Berberis vulgaris (B. vulgaris) is a thorny herb belonging to the family Berberidaceae found in different parts of the world. History of the application of this plant as a medicinal herb dates back to 3000 years (8). Due to containing alkaloid compounds, it has significant effects on the treatment of many diseases including leishmaniasis; it also prevents bacterial growth, reduces inflammation, stimulates bile secretion, reduces blood pressure, etc. (8, 9). Following laboratory analyses, various alkaloids of this plant were identified including berberine, oxycontin, jatrorrhizine, berbamine, etc. (8, 10, 11). Nisin is a polypeptide produced by some strains of Lactococcus lactis. The World Health Organization (WHO) permitted the application of nisin as a food preservative and it is currently used in more than 50 countries (12). Nisin has inhibitory effects against several bacteria (13). Sodium diacetate is a natural food preservative with the trade name Alvita that is permitted as a food additive by Food and Drug Administration and WHO and no poisonous effect is reported with its permitted values. Sodium diacetate is usually added to food products and uses as a growth inhibitor for molds (14). Escherichia coli are considered a poisonous factor in most foods. Several strains of E. coli are identified as potential foodborne pathogens of which E. coli O157:H7 is one of the most important ones that can cause gastrointestinal symptoms in human. It is a well-known serotype of enterohemorrhagic E. coli (EHEC) and the main cause of hemorrhagic colitis and hemolytic uremic syndrome (HUS) in human (15, 16). Occurrence of resistance against chemical microbial agents and their devastating effects paved the way to use herbal medicines as food preservatives or treatment of infectious diseases. On the other hand, their synergistic effects in concomitant use of various antimicrobial agents may reduce the administered doses of drugs and preservatives.
2. Objectives
The current study aimed at investigating the antimicrobial effect of the F. vulgare and B. vulgaris extracts individually and combined with nisin and sodium diacetate on E. coli O157:H7.
3. Methods
3.1. Preparation of Extracts and Chemical Analysis
Barberry root (B. vulgaris) and fennel seed (F. vulgare Mill.) were collected and the taxonomic identification of plant materials was performed by the Herbarium of Medicinal Plants, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. Extracts of the barberry root and fennel seed were prepared by maceration in 80% ethanol (Merck, Germany). The extracts were then condensed and dried by rotary and freeze drying machines, respectively. Fennel seed extract was analyzed by gas chromatography-mass spectrometry (GC-MS) and barberry root extract was analyzed using high-performance thin-layer chromatography (HPTLC) (7, 17). In the current study, silica gel 60 F254 was employed for the stationary phase studying and butanol (Merck, Germany): acetic acid (Merck, Germany): water (8:1:1) solvent system for mobile phase. Then to standardize the extract, serial dilution of berberine in methanol solvent was prepared and the absorbance of the solution was recorded at 346 nm wavelength by a spectrophotometer and the calibration curve was drawn. Also, a standard concentration of the barberry extract in methanol was prepared and its absorption was measured in the calibration curve of berberine (17).
3.2. Bacterial Strain, Media, and Supplements
Lyophilized E. coli O157:H7 (ATCC 43895) was purchased from Pasture Institute of Iran (Tehran, Iran). It was cultured at 37ºC in brain-heart infusion (BHI) broth (Merck, Germany), and stored at -20ºC with 10% glycerol. Mueller-Hinton agar (MHA) and Mueller-Hinton broth (MHB) media were obtained from Quelab Chemical Co. (Canada). Nisin (2.5% w/w) and sodium diacetate were purchased from Sigma Co. (the USA). Also, 2.5% pure nisin was suspended at 20 mg/mL in MHB containing 0.02 M HCl for activation (containing 500 µg/mL pure nisin); then it was serially diluted to investigate the antibacterial activity.
3.3. Disk Diffusion Test
Disk diffusion method was employed to examine the antibacterial activity according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) (18). For this purpose, 30 µL of each extract (12 mg/disk), nisin (3, 7.5, and 15 µg/disk) and sodium diacetate (12 and 24 mg/disk) were put on standard sterile blank disks (Padtan Teb, Iran) with 6 mm diameter and after drying aseptically, were placed on the inoculated MHA with 0.5 Mcfarland turbidity of the bacterium. Some standard antibiotic disks (i.e., erythromycin, gentamicin, streptomycin, and ciprofloxacin) were employed as positive controls and sterile distilled water as well as the solvent of extracts (Tween 80) as negative controls. Then, the inoculated plates were incubated at 37ºC for 24 hours and the growth inhibition zones were measured in millimeter to evaluate antibacterial activity. All the experiments were performed in triplicate.
3.4. Broth Microdilution Test
Broth microdilution method was employed to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC), according to CLSI guidelines in triplicate, with some modifications (19, 20). Bacterial inoculants (5 × 106 CFU/mL) were prepared with two overnight cultures at 37ºC for 20 hours; then a serial dilution was prepared to enumerate bacteria and afterwards, each dilution was plated on MHA in triplicate. Broth microdilution was performed in 96-well microplates with consecutive dilutions of the extracts (0.2 to 360 mg/mL), nisin (0.12 to 400 µg/mL), and sodium diacetate (10 to 720 mg/mL) in MHB and then a certain amount of the initial bacteria was inoculated into each well. Firstly, different concentrations of the barberry root and fennel seed extracts were set up in MHB supplemented with 2% Tween 80 (Samchun, South Korea) as the solvent of the extracts. Then, 160 µL of MHB, 20 µL of different concentrations of the extracts, nisin or sodium diacetate, and 20 µL of the initial bacterial inoculum (5 × 106 CFU/mL) were added into each well to achieve a concentration of 105 CFU/well. Negative (uninoculated MHB) and positive (inoculated MHB without the extracts, nisin, or sodium diacetate) control cultures were also designed. Contents of the 96-well microplates were mixed on a plate shaker and incubated at 37ºC for 24 hours. The MIC is defined as the lowest concentration that prevents the growth of the bacteria. Therefore, the concentration of the first well without turbidity was considered as MIC. The MBC is the lowest concentration of the solution required to kill bacteria. To determine combination effects, the abovementioned procedure was employed, but 140 µL of MHB, 20 µL of different concentrations of nisin (0.005 - 100 µg/mL), or sodium diacetate (0.25% - 56%) plus 20 µL of equivalent to 1/2 MIC of the extracts and 20 µL of the bacterial inoculum were added to each well.
3.5. Statistical Analysis
All data were expressed as mean ± standard deviation (SD) and analyzed using SPSS version 16. One-way ANOVA and the Duncan test (P value < 0.05) were employed to examine the effect of various factors on E. coli O157:H7.
4. Results
The chemical composition of the barberry root and fennel seed extracts was analyzed by HPTLC and GC-MS. According to the obtained results, the main component was berberine in the barberry root (B. vulgaris) extract. Also, bis (2-ethylhexyl) phthalate and trans-anethole were the main components in the fennel seed (F. vulgare Mill.) extract. The results of antibacterial activity of the extracts, nisin, and sodium diacetate on E. coli O157:H7 based on the disk diffusion test results are presented in Table 1. The antibacterial activity of the extracts (MIC and MBC), individually and combined with nisin and sodium diacetate, based on the results of broth microdilution test are presented in Tables 2 and 3, respectively. Lower amounts of MIC and MBC indicate higher antibacterial activity. Statistical analysis using one-way ANOVA indicated a significant difference between antibacterial activities of the extracts against E. coli O157:H7. In comparison of the individual effects of studied factors, nisin showed the highest antibacterial activity followed by barberry root extract, fennel seed extract, and sodium diacetate. The findings conformed to the results of the agar diffusion test. The effect of sodium diacetate combined with the studied extracts on E. coli O157:H7 showed significant differences with its individual effect (P < 0.05). The combined effect of sodium diacetate and the B. vulgaris extract was stronger. Regarding nisin, there was no significant difference between combined effects of nisin and the extracts in MIC, compared with that of the nisin individually on E. coli O157:H7 (P > 0.05), but in terms of MBC, a significant difference was observed. It indicated a more significant bactericidal effect of nisin combined with the extracts. The combined effect of nisin and the B. vulgaris extract in MIC showed a higher antibacterial activity. In MBC, the combined effect of nisin and fennel was higher on E. coli O157:H7 indicating their higher synergistic bactericidal activity, which may be due to the difference in the bactericidal mechanism of the extracts.
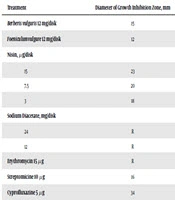
| Treatment | Diameter of Growth Inhibition Zone, mm |
|---|---|
| Berberis vulgaris 12 mg/disk | 15 |
| Foeniculumvulgare 12 mg/disk | 12 |
| Nisin, µg/disk | |
| 15 | 23 |
| 7.5 | 20 |
| 3 | 18 |
| Sodium Diacetate, mg/disk | |
| 24 | R |
| 12 | R |
| Erythromycin 15 µg | R |
| Streptomicine 10 µg | 16 |
| Cyprofluxazine 5 µg | 34 |
| Gentamicine 10 µg | 20 |
| Tween 80 30 µL | R |
Comparison of Antibacterial Activity of Studied Compounds and Standard Antibiotics Against E. coli O157:H7 Based on the Disk Diffusion Test Results
| Treatment E. coli | Nisin, µg/mL | Sodium Diacetate, mg/mL | Berberis vulgaris, mg/mL | Foeniculum vulgare, mg/mL |
|---|---|---|---|---|
| MIC | 0.41 ± 0.14 | 200.0 ± 0.0 | 40.0 ± 0.0 | 93.33 ± 23.09 |
| MBC | 20.0 ± 0.0 | 586.6 ± 46.1 | 200.0 ± 0.0 | 266.67 ± 23.09 |
Comparison of the Minimum Inhibitory Concentration and Minimum Bactericidal Concentration of the Extracts, Nisin, and Sodium Diacetate Against E. coli O157:H7 Based on Broth Microdilution Test Resultsa
| Treatment E. coli | Nisin, µg/mL + 1/2 MIC of Berberis vulgaris | Sodium Diacetate, mg/mL + 1/2 MIC of Berberis vulgaris | Nisin, µg/mL + 1/2 MIC of Foeniculum vulgare | Sodium Diacetate, mg/mL + 1/2 MIC of Foeniculum vulgare |
|---|---|---|---|---|
| MIC | 0.16 ± 0.07 | 53.3 ± 11.5 | 0.33 ± 0.14 | 80 ± 0.0 |
| MBC | 2.08 ± 0.72 | 120 ± 0.0 | 1.08 ± 0.14 | 560 ± 0.0 |
Comparison of the Minimum Inhibitory Concentration and Minimum Bactericidal Concentration of the Extracts Combined With Nisin and Sodium Diacetate Against E. coli O157:H7 Based on Broth Microdilution Test Resultsa
5. Discussion
It is evident that application of medicinal herbs has always been the oldest aproach towards treatment of diseases and during the developement of human civilization, there was always a close relationship between human and herbs. Since drug resistance and complications related to chemical antibacterial agents are rising, scientists’ attentions are drawn towards natural resources including antibacterial activity of herbs. Results of the current study indicated that nisin had the highest antibacterial activity against E. coli O157:H7, followed by extracts of B. vulgaris and F. vulgare Mill, while sodium diacetate had the lowest activity. Also, previous studies showed that B. vulgaris and F. vulgare Mill had antibacterial activities (21, 22). For example, Dashti et al., in a study on the antibacterial activity of B. vulgaris extract against some Gram-positive and -negative bacteria found that B. vulgaris has antibacterial activity, particularly against Pseudomonas aeruginosa, Proteus vulgaris, and E. coli (21). Also in a study, antimicrobial activity of some medicinal herbs was investigated on different microbial strains, in which the extract of F. vulgare Mill showed a growth inhibitory effect on Staphylococcus aureus, E. coli, and Salmonella typhi (23). Moreover, Saeidi et al., indicated antibacterial activity of the ethanolic extract of F. vulgare Mill against S. aureus, Bacillus subtilis, E. coli (PTCC:1074), and S. typhi. They observed that F. vulgare extract has the higher inhibitory effect on E. coli (22). In the current study, chemical analysis showed that the main component of the B. vulgaris extract is berberine. The antimicrobial activity of the B. vulgaris extract could be associated with berberine, although other compounds might be important. The mechanism of action of berberine is related to the inhibition of DNA, RNA, and protein synthesis, destabilization of the structure of bacterial cell membrane, and promotion or inhibition of enzymes activity (24, 25). It seems that bis (2-ethylhexyl) phthalate and trans-anethole, the main components of the F. vulgare Mill extract, are predominantly responsible for most of their antibacterial activities and pharmaceutical effects (26-28). Increased application of extracts or essential oils in oral products as antimicrobial agents can change their taste and flavor (29). Thus, preservation by multiple preservatives in fewer amounts is much better than the application of a high amount of one preservative (30). Therefore, to determine the efficiency of natural antibacterial agents, they should be examined individually and in combination with other antimicrobial agents to determine the synergistic effects. In many studies, synergistic effect between extracts, essential oils, and other antimicrobial agents is confirmed. For example, the antimicrobial activity of concomitant use of Mentha spicate and nisin against Listeria monocytogenes was investigated. The study indicated that their concomitant use led to a significant decrease in the number of bacteria (31). In a study, concomitant use of nisin and sodium diacetate as natural preservatives in French ketchups was investigated and it was found that nisin at 200 and 250 ppm and sodium diacetate at 750 ppm could keep the microbial load of the product within permissible range during preservation period and also it could maintain pH at standard levels (32). Also in a research, antimicrobial activity of nisin combined with Zataria multifora extract against L. monocytogenes was examined and the results showed that the inhibitory effect of Z. multifora obviously increased when used in combination with nisin (33). Moreover, a synergistic inhibitory effect was observed on E. coli O157:H7 when using a combination of monolaurin and spearmint oil (34). In the current research, synergistic inhibitory effect of the extracts combined with nisin or sodium diacetate on E. coli O157:H7 was obtained. Therefore, the studied extracts could be recommended, especially in combiation with other antimicrobial agents such as nisin and sodium diacetate, as pharmaceutical materials to develop new antimicrobial agents.