1. Background
P-glycoprotein (P-gp) is a 170-kDa transmembrane glycoprotein. This protein is encoded by the MDR1 (ABCB1) gene on the human chromosome 7p21. P-gp overexpression has been associated with multidrug resistance (MDR) in cancer cells (1, 2). This overexpression is responsible for intrinsic and acquired drug resistance in different human cancers (3). This overexpression can reduce intracellular anticancer-drug concentration as is frequently associated MDR in human cancer cells (4). Conversely, knockout mice lacking the P-gp gene show increased drug sensitivity (5). There are reports indicating the effect of anticancer drugs influencing transcriptional and post transcriptional mechanisms of the P-gp in different normal tissues (6-8). Our knowledge is limited about the details of how these drugs interact with the P-gp. The effect differs probably in different cancer types. HCC is one of the most common cancers affecting more than one million individuals leading to over 260000 deaths annually, worldwide. Although, the chemoprevention is consequently one of the effective ways to cure cancerous liver tissue (4), a major concern is potential of drug efflux transporter expression, which can significantly affect treatment efficacy. Although the main strategy for the treatment of HCC is systemic chemotherapy, higher levels of P-gp expression adversely affect the efficacy of chemotherapy (9) and this higher P-gp expression has a tendency to produce resistance to anticancer drugs. Therefore, we hypothesized that down-regulation of P-gp may enhance the effectiveness of chemotherapy. Distribution of rat mdr1a mRNA has been shown to be lower compared to the mdr1b mRNA in the liver tissue. Therefore, to better contrast in the quantitative expression analysis, we limited the study to the mdr1a mRNA.
2. Objectives
The present study aimed to investigate the role of verapamil and rifampin on P-gp expression level in HCC.
3. Materials and Methods
3.1. Animals
Thirty adult male albino rats (body weight range of 180-200 grams) were obtained from the central laboratorial animal facility at the Faculty of Medicine of Jundishapur University, Ahvaz, Iran. Rats were housed in individual metabolic cages under controlled environmental conditions (25˚C and a 12-hour light/dark cycle). Rats had access to pulverized standard rat pellet food and tap water ad libitum.
3.2. Material
NDEA (Sigma Aldrich, USA) was dissolved in saline and administered in a single dose (200 mg/kg i.p) to induce hepatic cancer. Rifampin and verapamil were purchased from (Sobhan Daro Co. Iran).
3.3. Experimental Design
HCC was induced using Nitrosodiethylamine (NDEA) in rats as a similar and realistic model in human (10). NDEA is an N-nitroso-alkyl compound and a well-known potent hepatocarcinogenic agent (11). It causes perturbations in nuclear enzymes involved in the DNA replication and is normally used as a carcinogen to induce HCC in animal model (12). Thirty rats were divided into six groups (5 rats in each group) as follows: control group without any treatment, NDEA, NDEA + verapamil, NDEA + rifampin, a group receiving verapamil and a group rifampin. NDEA was administrated intraperitoneally in a single dose. Verapamil (25 mg/kg) (13) and rifampin (10 mg/kg) were orally administrated (14) from 13th to 15th days after the NDEA administration. Then, rats were euthanized and liver samples were immediately collected. This study was approved by the Institute Ethics Committee of the Faculty of Veterinary, Shahid Chamran University, Ahvaz, Iran.
3.4. Gene Expression Assay by Quantitative PCR (qPCR)
Total RNA was extracted from 30 mg rat liver sample, which was previously immersed in 1 mL of RNA-later using total RNeasy plus Minikit (Qiagen, Germany). The RNA concentration was measured by Nanodrop (Thermo Fisher, USA) after treatment with RNase free DNase (Qiagen, Germany). cDNA was immediately prepared from 1µg of total RNA using the high-Capacity cDNA Reverse Transcription Kit (Qiagen, Germany). For the real time PCR, rat mdr1a gene (Gene ID 170913) and GAPDH gene primers and probes (GenBank accession number NM-017008) were designed with Genscan software (Qiagene) as follows: 5-GGC CTC CAA GGA GTA AGA AA-3 as forward and 5-GGA ATT GTG AGG GAG ATG CT-3 as the reverse primer creating a 150 bp fragment with the TaqMan probe 5-fam-CCA CCC AGC CCA GCA AGG AT-tamra-3. The mdr1a gene was selectively amplified with the forward primer 5-CAG AAC GCA GAC TTG ATC GT-3 and the reverse primer 5-ATC TCG CAT GGT CAC AGT TC-3, which generates a 133 bp product with the labeled TaqMan probe 5-fam-TCA AGG AGC ACG GCA CCC AC-tamra-3. qPCR was performed in a 96-well reaction plate using the TaqMan Gene Expression Assay system (Applied Biosystems, model plus one, USA). Each reaction was performed in duplicate together with a negative control in which the reverse transcriptase enzyme was replaced with nuclease-free water. PCR reactions were performed totally in a total volume of 25 µL mix (qPCR probe master, Jena Bioscience, Germany) containing 2 µL of the cDNA template, 4 pmol of each primer, 2 pmol of probes and 0.25 µL ROX reference dye. The PCR conditions were as follows: initial denaturation at 95˚C for 1 minute, followed by 40 cycles of denaturation at 95˚C for 15 seconds, annealing at 57˚C (mdr1a and GAPDH) for 1 minute, and extension at 60˚C for 30 seconds. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a housekeeping gene was used for normalization of gene expression. Quantification of target gene expression was conducted according to the guideline and instruction for real time PCR systems (Applied Biosystems, USA). Standard curves for the target and housekeeping (endogenous control) genes mdr1a and GAPDH were constructed by using 10-fold serial dilution of rat cDNA. Negative controls with the omission of reverse transcriptase and the replacement of RNA with water were run with each set of reactions. Comparative threshold cycle (CT) quantification was used to calculate differential mRNA. CT values were initially standardized by the reference to the housekeeping gene GAPDH. The ΔCT values were computed as the difference between the mean values for the mdr1a gene and the mean of the CT values of GAPDH gene. Mean fold changes were estimated between the groups using the ABI software (Applied Biosystems, USA) (the standard curves were addressed previously in Ghasemian et al.) (15).
3.5. Statistical Analysis
Data was expressed as mean standard deviation. Statistical packages for social sciences (SPSS) (SPSS UK Ltd, Woking, Surrey, UK) was used for data analysis. Chi-square analysis was used to assess significant changes between different groups. The level of significance was set at P ≤ 0.05 and analysis of variance (one-way ANOVA) was used to compare the means of different groups.
4. Results
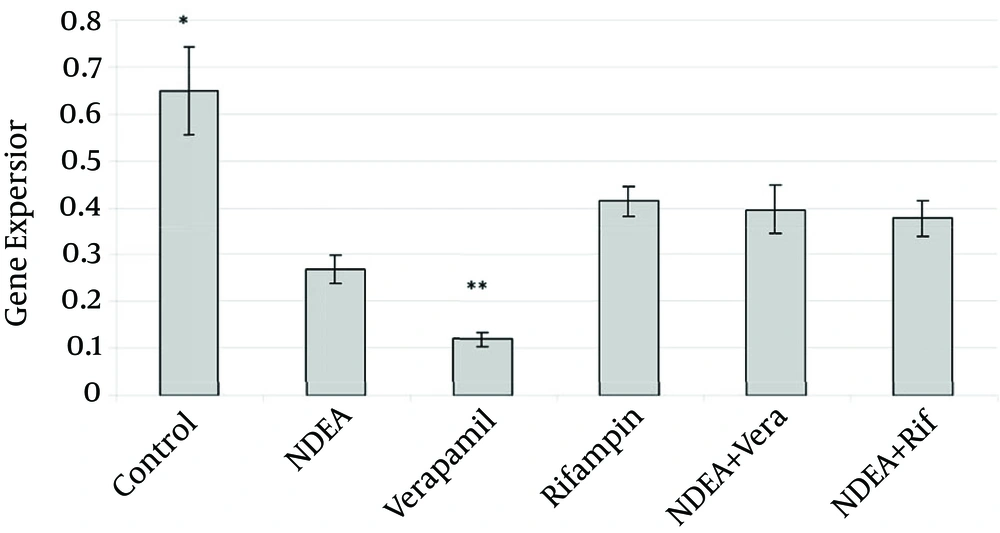
The average mRNA level (n = 5) of the mdr1a gene of the control group in the qPCR experiment was 0.6488 ± 0.2. This average was 5.4 fold higher than the verapamil administered group (0.1192 ± 0.03) and 2.4 fold higher compared to the HCC induced group (0.2685 ± 0.05) (P < 0.001). Rifampin treated group showed 1.5 fold lower (0.41375 ± 0.06) as the control group (P value ≤ 0.006). A combined NDEA and verapamil group indicated 1.64 fold lower (0.3954 ± 0.11) (P ≤ 0.002) mRNA level. Further, the mRNA level of the mdr1a gene in the control group was 1.72 fold higher (0.3766 ± 0.08, P ≤ 0.001) than the group with the NDEA-Rifampin treatment. Verapamil treatment group showed 3.47, 3.31 and 3.15 fold lower than rifampin, the NDEA+ verapamil (P ≤ 0.001) and the NDEA + rifampin administrated groups (p ≤ 0.002), respectively. There was no significant difference (P ≥ 0.05) between the NDEA + verapamil and NDEA + rifampin groups. In Figure 1, all the results were summarized. The results were shown as mean and standard deviation.
5. Discussion
In the present study, we investigated the effect of rifampin and verapamil on the mdr1a gene at the mRNA level in rat. The same effect was monitored in HCC induced rats by quantitative real time PCR. Interestingly, verapamil administration decreased the P-gp expression (P ≤ 0.001). The results indicated that expression levels of P-gp in groups receiving NDEA and verapamil together significantly reduced compared to the control groups. This expression level was higher compared to verapamil alone. The amount of gene in the group receiving NDEA and verapamil together was higher than the control group, therefore, a significant reduction in groups receiving NDEA and verapamil has been relatively expected and confirms results by recipient alone groups. On the other hand, according to the physiological role of this exogenous transporter protein, as well as the conversion of active metabolites in the liver, increased expression in the group receiving NDEA together with verapamil compared to the group receiving verapamil alone is expected. The results also showed a higher inhibitory effect of verapamil on the P-gp expression than rifampin. So far, many studies have been performed on the effects of cancer drugs on P-gp expression and its effects on drug resistance in different cancers as well as the effect of other drugs in healthy tissues. Different studies have not come to the same conclusions. This difference might be due to various drugs effects on P-gp expression in different tissues and cancers. In earlier studies, changes were shown in gene expression at the protein level. In the present study, the effects were compared at mRNA levels. Recent reports suggested the ability of verapamil to increase the production of free radicals leading to cell apoptosis (16, 17). We proposed that down-regulation of the mdr1a gene expression by verapamil is probably related to the general interruption of some molecular mechanisms due to initiation of cell apoptosis. Previous reports proposed verapamil as a possible P-gp substrate (18, 19). It was shown that the main mechanism for overexpression of P-gp in liver tumors (20), during liver regeneration (21) and during the establishment of cultured hepatocytes is increased mRNA stability. We conclude that verapamil might influence the P-gp at both message and protein levels, at least in HCC. Furthermore, we reported here for the first time the down-regulation of the P-gp mRNA in rat liver by rifampin administration. The results showed that gene expression was significantly reduced in the group receiving rifampin compared to the control. In controversy, Larsen et al. reported that rifampin increased the P-gp level at both message and protein levels in human colon tissue (22). Some investigations on human cells showed the same results (23, 24). One of the main reasons for these differences could be due to the effect of rifampin on the expression of mouse P-gp protein. This finding might be due to different interactions between rifampin and MDR transporter system. It is considered that binding of rifampin to some members of transcription factors, such as the pregnant x receptor (PXR), causes translocation of the mentioned factors from the cytoplasm to the cell nucleus, which activates the transcription of distinct genes (16, 17). Ott et al. described that rifampin acts as a legend for the Pregnant X receptor (PXR) in human and pig (25). We speculate that interspecies differences might be the reason why rifampin did not interact with rats PXR family. The hypothesis needs more evaluation in further investigations. This result may demonstrate that MDR-1 expression is a poor drug resistance indicator. Furthermore, if higher levels of P-gp expression adversely affect the efficacy of chemotherapy, verapamil and rifampin are strong P-gp expression inhibitors in hepatocellular carcinoma. Therefore, if drug transporters are important resistance mechanism in HCC, these drugs are effective in HCC chemotherapy. In conclusion, these in vitro experimental model studies suggest the possibility to use verapamil and rifampin for HCC by inhibiting P-gp mRNA overexpression in liver. These data support the need for in vivo trials.