1. Background
Inflammation is the body’s response against invading pathogens, which is typically characterized by redness, swelling, pain, and heat. Several reports have provided evidence that inflammation is involved in the pathogenesis of many diseases, including aging (1), cancer (2), atherosclerosis (3), cardiovascular disease (4), arthritis (5), and neurodegenerative diseases (6). Current anti-inflammatory drugs, despite their proven efficacy in alleviating symptoms and providing pain relief, have considerable adverse effects including gastrointestinal problems and renal damage (7). Medicinal plants are believed to be important sources of new chemical substances with potential therapeutic efficacy. Considering that the most important anti-inflammatory drugs (e.g. salicylic acid) were originally derived from plant sources, the study of traditionally used plant species should be seen as a useful research strategy in the search of new drugs. The palm tree belongs to a family of monocotyledons plants known as Palme, Palmacea, or Areacacea that consists of a great number of species (8) including 189 genera, which are classified into five subfamilies (9). Washingtonia is a genus of palms belonging to the Coryphoideae subfamily (Coryphieae tribe and Livistoninae subtribe) and includes two species: Washingtonia filifera and Washingtonia robusta. They differ in subtle characteristics, and even palm experts have trouble to distinguish them. Washingtonia filifera known as California fan palm, desert fan palm, or Washington palm is the only palm native to California and considered as the largest one in the United States. Fruits and seeds of W. filifera were used as a food resource by the Cahuilla Indians of the southern California deserts (10). In Iran, this plant is widely found in Khuzestan province. The percentage composition of the W. filifera seeds are as follows: ash, 1.37%; oil, 16.30%; protein, 3.46%; total carbohydrate, 77.19%; and moisture, 3.22%. The major nutrients (mg/100 g of seeds) found in the seeds are: potassium (67.33 mg/100 g), magnesium (34.35 mg/100 g), calcium (187.85 mg/100 g), and phosphorus (23.26 mg/100 g) (11). The total unsaturated fatty acid of W. filifera seed oil is 57.39%. It can influence the physical properties of the membrane such as nervous cell construction (12). Moreover, W. filifera is characterized by its high content of flavonoids, mainly 8-hydroxyisoscoparin, luteolin 7-O-glucoside 4''-sulfate, and luteolin 7-O-glucoside 2''-sulfate. El-Sayed et al. showed the antioxidant activities of two new flavonoids isolated from W. filifera (13). Flavonoids exist in a large group of plants which are characterized by a diphenylpropane structure (C6-C3-C6). They are widely distributed throughout the plant kingdom and are commonly found in fruits, vegetables, and certain beverages. Numerous preclinical and some clinical studies suggest that flavonoids have potentials for the prevention and treatment of several diseases (14, 15). Furthermore, they have shown different pharmacologic effects including anti-inflammatory and antinociceptive effects (16-18), improvement of learning behavior (19), and antioxidant, anti-aging, and antineoplastic characteristics (13, 20, 21).
2. Objectives
Regarding these particular features and other desirable physicochemical characteristics, the value of W. filifera seeds extract (WSE) in health medicine may be justified. On the other hand, carrageenan-induced rat paw edema is a widely used test to determine anti-inflammatory activity, which constitutes a simple and routine animal model for evaluation of pain at the site of inflammation without any injury or damage to the inflamed paw (22). Present study mainly aimed to investigate the role of intraperitoneal (i.p.) administration of hydroalcoholic (WSE) in alleviating peripheral inflammation of carrageenan-induced rat paw edema. The current study would help to substantiate the traditional uses of WSE and provide an alternative therapeutic medicine to the current anti-inflammatory drugs.
3. Materials and Methods
3.1. Plant Material and Preparation of the Extract
Plants were collected from Ahvaz. Washingtonia filifera was identified by Ahvaz Jundishapur University of Medical Sciences (AJUMS), Ahvaz, Iran, and voucher samples were preserved for reference in the herbarium of School of Pharmacy, Ahvaz, Iran (A14012001P). To prepare hydroalcoholic extract of seeds, powdered seeds (100 g) were macerated by 1500 mL of 70% ethanol (v/v) for 72 hours. The extract was then shaken and filtered, and the solvent was removed in a vacuum evaporator to obtain semisolid extract and then was placed in an oven in 60°C for 72 hours (23).
3.2. Drugs
Indomethacin and carrageenan were purchased from Sobhan Pharmaceutical Co (Tehran, Iran) and Sigma Chemical Co (St. Louis, MO, USA), respectively. The WSE and drugs were diluted in saline.
3.3. Animals
Male Wistar rats weighing 150 to 180 g were obtained from a random bred colony in the animal house of AJUMS. Animals were housed in standard cage with 12-hour light/dark cycle and air temperature was maintained at 22°C ± 2°C. Experiments reported in this study were performed in accordance with local guidelines for the care of laboratory animals of AJUMS.
3.4. Experimental Groups
Acute edema was induced in the right hind paw of rats by injecting 100 µL of 1% carrageenan solution after 30 minutes of vehicle or WSE (100, 200, and 400 mg/kg, i.p.). The doses of WSE were selected based on pilot experiments in our laboratory. The reference drug, indomethacin (10 mg/kg, i.p.), were also used for comparison (16). Carrageenan was injected under the plantar region of right hind paw, and the volume was measured using a plethysmometer (UGO Basile, Italy) at hours one, two, three, four, and five of carrageenan challenge. Inflammation was expressed as change in paw volume (24).
3.5. Statistical Analysis
All experimental results are given as means ± for six to eight animals per group. Curves were constructed by plotting the change in paw edema as a function of time. The area under the paw volume versus time curves (AUC), an expression of the duration and intensity of the effect, was calculated using the trapezoidal rule. The changes in paw edema were converted to percentage of maximum possible effect (%MPE) as follows: %MPE=(Paw volume, saline control - Paw volume, test drug) / (Paw volume, saline control) ×100. The median effective dose (ED50) value, the dose of seed extract reducing the nociceptive response by 50% relative to the control value, was reported as geometric mean accompanied by its respective 95% confidence limit. The ED50 value was determined by linear regression from individual experiments using GraphPad software (GraphPad Prism 5, San Diego, CA, USA). The statistical analyses were performed by one-way ANOVA followed by Tukey’s post hoc test. A two-way ANOVA followed by Bonferroni’s test was carried out for the time-course effect of WSE.
4. Results
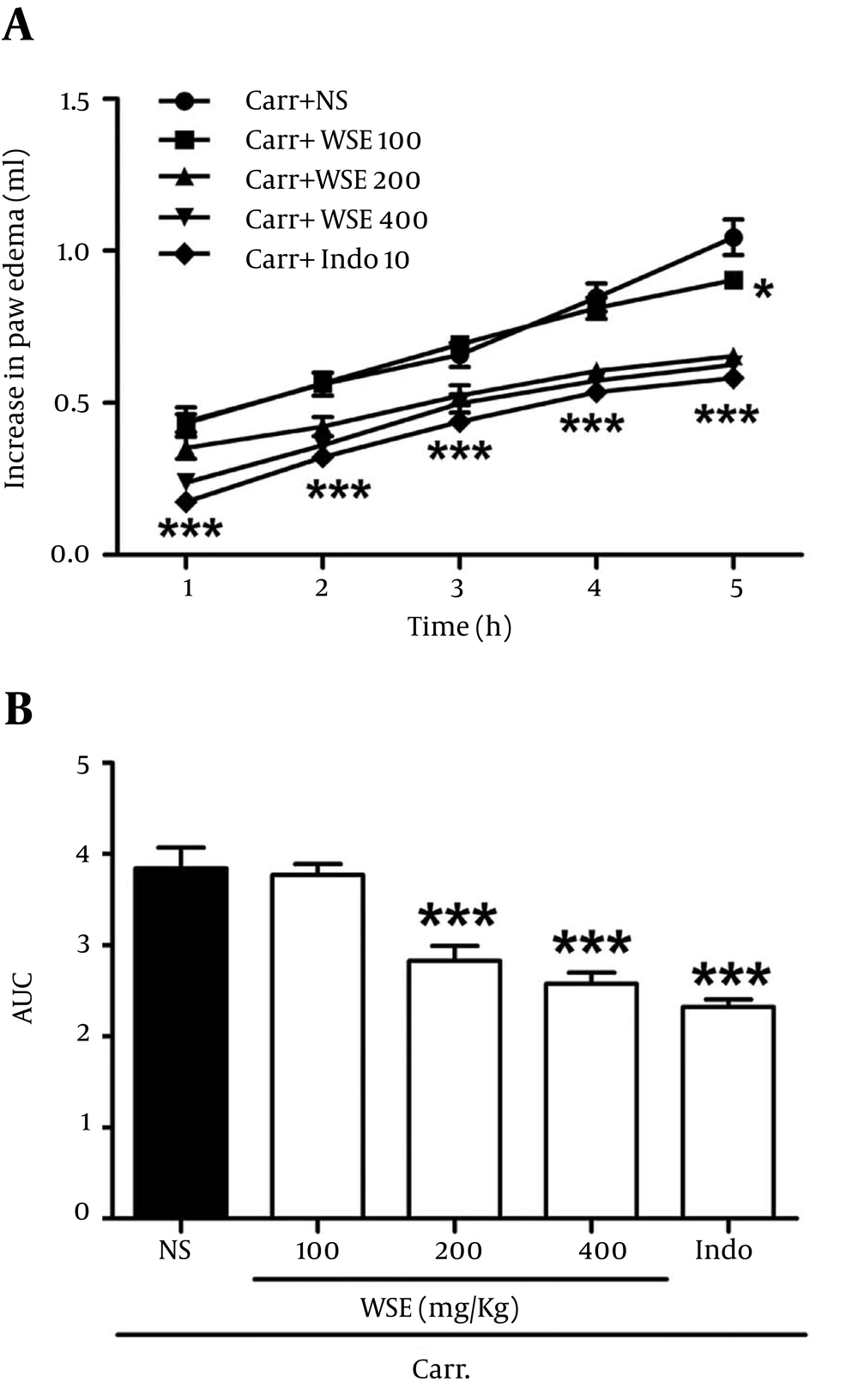
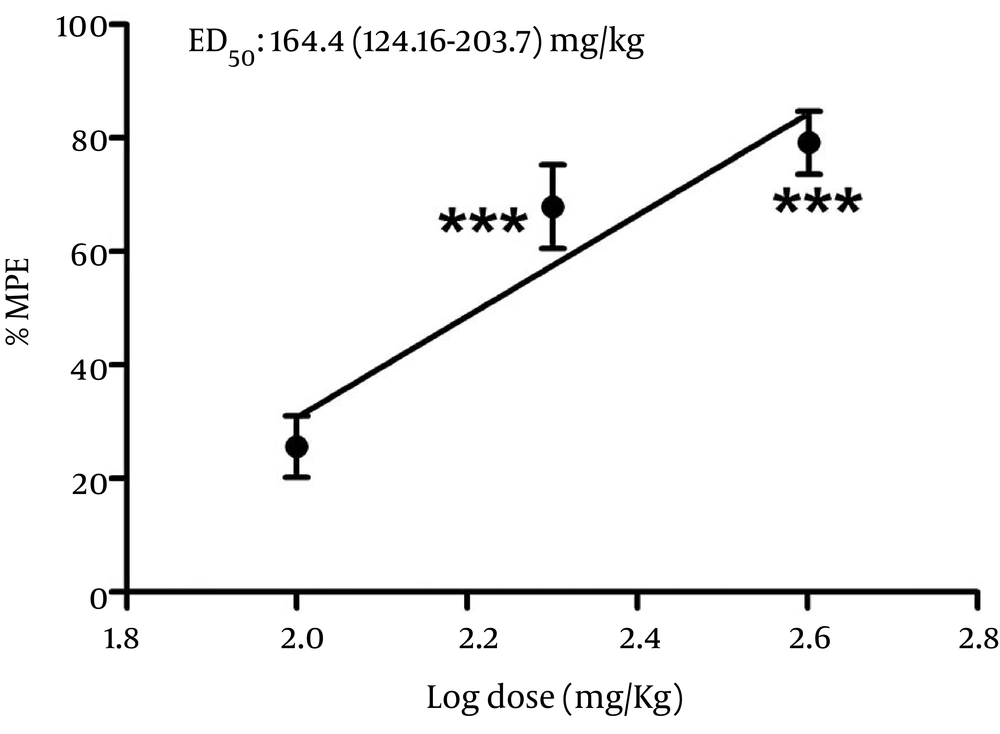
Injection of carrageenan (1%, 100 μL) into the ventral surface of the right hind paw evoked a characteristic edematogenic response in animal model. According to Figure 1A and 1B, i.p. indomethacin (10 mg/kg) reduced the edema volumes in comparison to control group (Carr + NS) during the five hours of carrageenan treatment. Further, in the range of 100 to 400 mg/kg, WSE showed a dose-dependent inhibition of edema development after five hours of carrageenan treatment with percentage of inhibition of 25.6%, 67.9%, and 79.2%, respectively. In addition, the anti-inflammatory effect of WSE was comparable to indomethacin (the positive controls). Furthermore, the calculated mean ED50 for i.p. administration of WSE was 164.4 mg/kg (95% CI, 124.16-203.7 mg/kg) as shown in Figure 2.
A, Time course of anti-inflammatory effect of Washingtonia filifera seeds’ hydroalcoholic extract (WSE) and indomethacin (Indo, 10 mg/kg) in the carrageenan test; B, Data are expressed the area under the paw volume versus time curves (AUC). Each value represented as mean ± S. E. M. *P < 0.05 and ***P < 0.001 as compared with the Carr + NS group.
Ordinate shows percentage of anti-inflammatory effect after Washingtonia filifera seeds’ hydroalcoholic extract (WSE) administration. All points represent the mean ± S. E. M (n = 6-8). Values of median effective dose (ED50; 95% CI) are presented on the figure. The asterisks denote the significance levels when compared with the control groups (P < 0.001).
5. Discussion
The acute inflammatory response is a series of local cellular and vascular responses that occurs immediately following tissue damage, and this biological response is a protective mechanism of body to remove the injurious stimuli, such as pathogens, irritants or physical injury, from the tissues and to initiate the healing process (25). The present study intended to evaluate the anti-inflammatory effect of WSE on the carrageenan-induced inflammation in rats. The carrageenan test is highly sensitive to nonsteroidal anti-inflammatory drugs, and has long been accepted as a useful model to determine the anti-inflammatory effects of natural products (25, 26). The development of edema in the rat hind paw following the injection of carrageenan has been described as a biphasic process, in which various mediators operate consecutively to produce the inflammatory response. Histamine, serotonin, and bradykinin are the first detectable mediators in the early phase of carrageenan-induced inflammation. Prostaglandins are involved in the increased vascular permeability, and are detectable in the late phase of inflammation. In addition, local and/or systemic inflammation is associated with enhanced levels of the proinflammatory cytokines TNF-α, IL-1, and IL-6 (27). On the other hand, the carrageenan-induced inflammatory response has been linked to neutrophils infiltration and the production of neutrophils-derived free radicals (28).
Our experimental results showed that intraplantar administration of carrageenan increased paw volume significantly. The genus Washingtonia is indigenous to California but has been cultivated in Iran and elsewhere. Previous phytochemical study of this species detected lipids, proteins, leucoanthocyanins, flavonols, C-glycosylflavones, and flavonoid sulfates (29-31). While the flavonoid sulfates are not widely distributed in the plant kingdom, they occur in many members of the Palmae, especially in important palm genera such as Washingtonia (32). The sulfated flavonoids from W. filifera were reported to impart antimicrobial activity (33). The results of the present study clearly indicate that administration of WSE (200 and 400 mg/kg) effectively reduced the carrageenan-induced inflammation in dose-dependent manner, which was comparable to that of indomethacin (10 mg/kg; i.p.). Although the precise site and mechanism of the anti-inflammatory effect were not addressed in the present investigation, it might be due to antioxidant activity and/or increase in the activities of antioxidant enzymes. Velioglu et al. reported the antioxidant activity of W. filifera extracts (21). Furthermore, recent studies have shown that action of some phenolic compounds such as crocin (34, 35), rutin (36), and resveratrol might be related to their antioxidant properties (37).
It has been reported that polyunsaturated fatty acid have important roles as mediators and regulators of inflammation. Nehdi et al. showed that the total unsaturated fatty acid of W. filifera seed oil is 57.39%. The most important composition are oleic, linoleic, and lauric acid (11). Unsaturated fatty acid can influence the biochemical properties of the membrane such as fluidity and permeability (12). It has been shown that incremental consumption of polyunsaturated fatty acids results in a partial replacement of the arachidonic acid in cell membranes by eicosapentaenoic and docosahexaenoic acids. This leads to decreased production of arachidonic acid-derived mediators and therefore, is a potentially beneficial anti-inflammatory effect of unsaturated fatty acids. Also, unsaturated fatty acids have a number of other effects that might occur at the downstream of altered eicosanoid production or are independent of this. For example, they result in suppressed production of proinflammatory cytokines and can modulate adhesion molecule expression (38). Therefore, according to previous evidence on the anti-inflammatory effects of unsaturated fatty acid, it could be a possible mechanism for anti-inflammatory effect of WSE (39, 40).
The present results demonstrate that WSE exerts a potent action against inflammation and confirm that the extract contains an effective anti-inflammatory substance(s). Further studies are required to characterize the exact mechanism(s) responsible for the anti-inflammatory effect.