1. Background
A wound is a physical injury that arises from an opening or breaking of the skin. Wound healing takes place over a dynamic and complex process that comprises of a series of regulated events including inflammation, tissue formation, revascularization, and tissue remodeling. Throughout the healing process, several growth factors are secreted to expedite wound healing (1). Optimal wound healing occurs when tissue damage is minimized by providing adequate tissue perfusion and oxygenation, proper nutrition, and a moist environment to restore the continuity and function of the affected area (2, 3). Numerous researchers have investigated the medicinal properties of herbs on animal by using both excisional and incisional models (4, 5). Recently, development of new drugs with herbal origin is considered for the decline of chemical drugs due to side effects (6). In folk knowledge, medicinal plants are used for treatment of various disorders with little information of their pharmacological uses (7). Quercus is the most frequent genus of the Fagaceae family in the forests of Iran (7). Several species of oaks grow abundantly in Zagros, Arasbaran and Hyrcanian forests exhibiting remarkable morphological variations. The main species of forests in Lorestan province is Q. brantii, which is widely distributed all over the area, particularly between 700 and 2400 m of altitude above the sea level. Quercus brantii popularly known as Persian oak in English and “Balout Irani” in Persian is used for treating gastric disorders by traditional people in Zagros region (Lorestan, Ilam and Kohghiluyeh and Boyer-Ahmad provinces), southwestern Iran (7, 8). The tannins extracted from Q. brantii have contractive and disinfectant effects (9). The extracts isolated from Q. brantii seed and seed hulls have been shown to have biological and pharmacological activities such as antibacterial (9, 10), and wound-healing effects (11-13). Various plant species have served as a source of medicine for people all over the world. For years, plant has been one of the most intense areas of natural product research yet the field is far from being exhausted. Plants and their extracts have immense potential for the management and treatment of wound. Phytomedicines for wound healing are not only cheap and affordable but also purportedly safe as hypersensitive reactions are rarely encountered with the use of these substances. These natural substances prompt healing and regeneration of the tissue by various means, however, scientific validation and standardization is needed.
2. Objectives
Persian oak (Quercus brantii) belongs to the Fagaceae family. It is a medicinal plant which is commonly used by the tribes in south western Iran to treat inflammatory and gastric ulcers. The aim of this study was to evaluate the effectiveness of Persian oak (Quercus brantii) ointment on dermal wound healing in rats.
3. Materials and Methods
3.1. Plant Material and Persian Oak Ointment Preparation
Fruits of Persian oak (Quercus brantii Lindl., the Fagaceae family) were collected in September 2013 from Ardal region in central Zagros mountains (latitude. 31° 50´, longitude. 51° 23´, altitude. 2120 m above sea level), Chaharmahal Bakhtiari Province, Iran. Plant identities were confirmed by Dr. H.A. Shirmardi, and a representative voucher specimen (IAUSHK-351) was placed in the herbarium of research center of medicinal plants and ethno-veterinary, Islamic Azad university, Iran. Seeds of Persian oak were dried inside for one week at room temperature (30°C ± 5°C), and were grounded to fine powder using the Moulinex food processor. The hydro-alcoholic extract was obtained by maceration of the crude plant powder with ethanol/water (70/30) for four days in a chamber temperature (35°C ± 5°C) in the dark. The extract was filtered using a sterile cloth sheet. The filtrate was evaporated under reduced pressure at temperature below 45°C with a rotary evaporator and a dark green hydro-alcohol extract (yield: 14.1%) was obtained. It was mixed with eucerin for preparing 1% and 2% ointments (w/w).
3.2. Animals
We used adult male albino rats (180 - 240 g) for our study. Before start of the experiment, they were kept for 7 days at controlled temperature (22°C ± 2°C) for acclimatization and during this time they had free access to food and clean drinking water. All the tests were done during the daylight. All the experiments were done in accordance with the US national research council guidelines on the use of care of laboratory animal resources and were approved by animal ethics committee of Ahvaz Jundishapur university of medical sciences.
3.3. Experimental Groups
The lesioned rats were divided into 5 groups, 8 rats each: group 1 received no treatment, group 2 received eucerin as the negative control, group 3 received 1% Persian oak ointment in eucerin base, group 4 received 2% Persian oak ointment and group 5 received 1% phenytoin in eucerin base as a standard healing agent.
3.4. Skin Ulcer Induction
A deep skin ulcer model was created using rats as follows (1, 6). The rats were anesthetized by intraperitoneal injection of xylazine hydrochloride (10 mg/kg) and ketamine hydrochloride (25 mg/ kg) and their back hair was shaved and the application field was outlined with a marking pen just prior to skin removal (Figure 1). A full-thickness wound in 1 cm diameter was made in each rat by removing skin of the dorsum with circularly motions. The tissues removed included the skin, subcutaneous tissue, fascia, and fleshy panniculus. Tools that we used were scalpel, straight scissors and Adson forceps (14). The wounds were left undressed exposed to the open environment. Eucerin, standard drug (1% phenytoin) and Persian oak ointment (1% and 2%, w/w) were applied topically twice a day to the control group, standard group and treatment group, respectively, till the wounds were completely healed.
3.5. Measurement of Wound Contraction
The wound surface area was measured on the first day of wound healing. The size of wound was drafted on a transparent sheet, during the monitoring process, and was then transferred to graph paper to be evaluated on the surface area (13). The extent of wound closure was measured as the ratio of wound area (each day after wound healing process) to the original wound size.
3.6. Histological Evaluation
Subsequently, macroscopic evaluation was performed. The excised tissue was restored with 10% buffered formalin. Each sample was inserted in a paraffin block and finely sliced at 2.5 µm, and stained with hematoxylin-eosin. A pathologist was called in the experiment to scrutinize the slides under light microscope to evaluate the severity of histopathological changes. Factors such as epidermal and dermal regeneration, granulation tissue thickness, and angiogenesis were scored as described by Priya et al. (15) according to the criteria (Table 1). It was considered angiogenesis only when vessels contained erythrocytes. To evaluate well formed from poorly formed capillary vessels, the following parameters were considered: presence or absence of edema, congestion, hemorrhage, thrombosis, and intravascular fibrin formation. Paraffin blocks were prepared from the wounded tissue and histological slides were obtained from the widest area of the wound.
3.7. Epithelialization Period
It was evaluated through counting the number of days required for the scar to fall regardless of the raw that was left behind.
3.8. Tensile Strength
The tensile strength is expressed by the degree to which a tissue shows integrity. The tensile strength increment represents better healing in wound area. At the end of the healing period, a strip of repaired tissue measuring 20 × 5 mm was cut off and the tensile strength measured with a tensiometer. Tensile strength was calculated using the following formula (14):

3.9. Hydroxyproline Estimation
Hydroxyproline is a rare amino acid found in the collagen fibers of granulation tissues. Estimation of hydroxyproline is aimed at understanding the progress rate at which the wound is healing in the connective tissue. Once complete healing had achieved, a piece of skin from the healed area was collected (100 mg) for analysis. Hydroxyproline was measured by the method used by Edwards and his colleague (16). Tissues were hydrolyzed in 6 M Hydrogen chloride HCl for 18 hours at 110°C. Samples were dried in hot air oven at 60°C - 70°C to a constant weight and then washed three times with distilled water. The acid-free samples were reconstituted in 2.0 mL of acetate-citrate buffer [1.2% sodium acetate trihydrate, 5% citric acid, 12% sodium acetate, 3.4% sodium hydroxide (pH 6.0)] and then, 500 mL of 0.05 M chloramine-T was added to 1 mL of sample, after which the samples were incubated for 20 minutes at room temperature, followed by the addition of 0.5 mL of 15% perchloric acid and 15% 4-dimethyl aminobenzaldehyde in 1-propanol. After incubation for 15 minutes at 60° C, each sample was transferred to a microtiter plate and absorbance was read at 550 nm using an ultraviolet-visible (UV/Vis) spectrophotometer. Hydroxyproline concentrations of the unknown samples were calculated from a linear standard curve and presented as μg/mg dry tissue weight.
3.10. Collagen Estimation
On day 1 and day 10, a piece of skin from the wound area was collected and analyzed for collagen content. Five milliliter of TES buffer and 0.1 mL of acid hydrolysate sample was added in the test tube, mixed well, and incubated at 37°C. After 5 hours, the contents were filtered through a syringe filter (0.8 µM) into clean containers and allowed to stand. For color development, 0.2 mL of test filtrate and 2 mL of ninhydrin color reagent (NCR) were added, mixed well, and placed in a boiling water bath for 30 minutes. After cooling to room temperature, 10 mL of 1-propanol was added to each container, mixed well, and the absorbance was read at 570 nm (17, 18).
3.11. Statistical Analysis
All experimental results are given as the mean ± standard error of mean (SEM). The statistical analyses were performed by one-way analysis of variance (ANOVA) followed by Tukey's post-hoc test unless otherwise stated. A two-way ANOVA followed by Bonferroni's test was carried out for the time-course effect of Persian oak ointment. Data analysis was performed using the Prism 5.0 (San Diego, CA, USA) statistical package program. P Values less than 0.05 were considered to be statistically significant.
4. Results
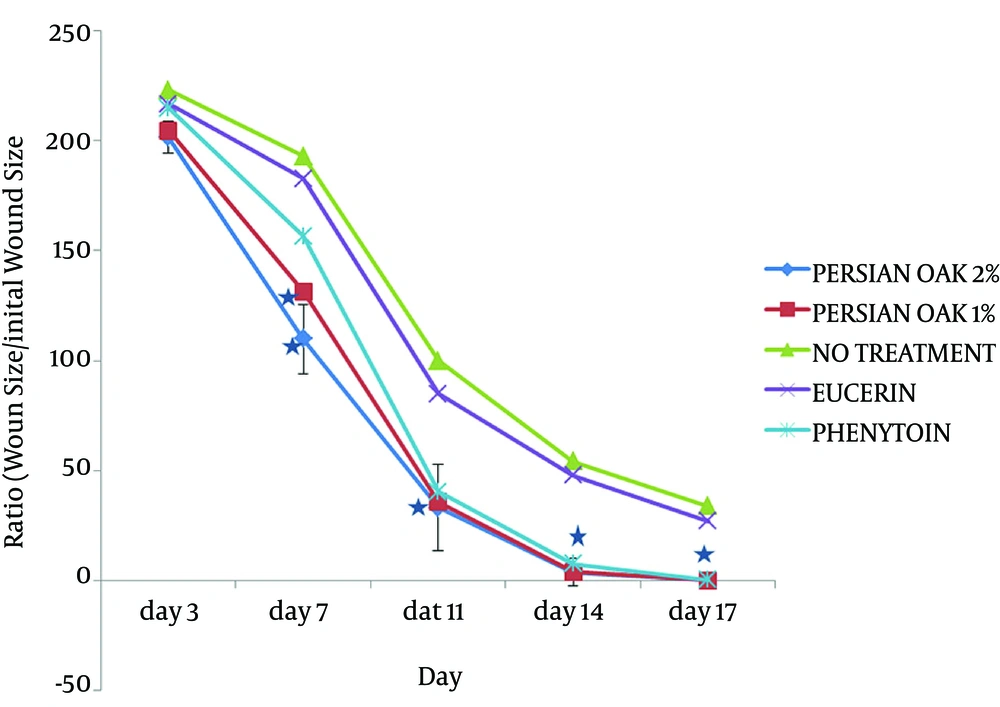
There was no significant difference in wound contraction between the nontreatment group and eucerin-treated group throughout the study period. Application of Persian oak ointment improved wound healing at all times beginning on the 3rd day with statistical significance (P < 0.05) achieved from 7th day as compared with the eucerin-treated group (Figure 2). However, no significant difference was observed in wound healing between Persian oak ointment 1% and 2% groups. Furthermore, results showed that phenytoin treatment was significant from the 10th day onwards.
The time taken for complete healing i.e. epithelialization time in the eucerin group was comparable to nontreated group. A statistically significant decrease was observed in epithelialization time in Persian oak ointment 1%, Persian oak ointment 2% (P < 0.01) and 1% phenytoin-treated (P < 0.05) groups as compared to the eucerin-treated group (Table 1).
The tensile strength of the group treated with eucerin was comparable with the nontreatment group. Tensile strength for Persian oak ointment 1%, Persian oak ointment 2% and 1% phenytoin at the end of the healing period was found to be significantly higher (P < 0.05) compared to the eucerin-treated rats (Table 2).
| Treatment Groups | Tensile Strength After 10 Days of Wound, g/cm2 |
|---|---|
| Nontreated group | 980 ± 36 |
| Eucerin group | 987 ± 33 |
| Persian oak ointment 1% | 1190 ± 39 |
| Persian oak ointment 2% | 1214 ± 32 |
| Phenytoin-treated | 1229 ± 60 |
The Effect of Persian Oak (1% and 2% w/w) Ointment; Phenytoin (0.1%) Ointment and Eucerinon Tensile Strengtha
Moreover, an increase in hydroxyproline content in Persian oak ointment 1%, Persian oak ointment 2% and 1% phenytoin-treated (P < 0.05) groups was observed to be significantly higher (P < 0.05) than the eucerin and nontreatment groups (Table 3).
| Treatment Groups | Hydroxyproline Content Tissue, μg/g |
|---|---|
| Nontreated group | 868 ± 3 |
| Eucerin group | 878 ± 18 |
| Persian oak ointment 1% | 1075 ± 40 |
| Persian oak ointment 2% | 1105 ± 34 |
| Phenytoin-treated | 1239 ± 38 |
The Effect of Persian Oak (1% and 2% w/w) Ointment, Phenytoin (0.1% w/w) Ointment and Eucerin on Hydroxyproline Content
4.1. Collagen Estimation
In the present study, collagen level of the wounded regions were found to have increased in groups treated with Persian oak ointment 1% and Persian oak ointment 2% versus negative control which implies more collagen deposition in treated groups than the control group (Table 4). Significant differences were observed in collagen concentrations between the Persian oak ointment 1% and Persian oak ointment 2% groups compared to the eucerin group (P < 0.01).
| Treatment groups | 1th day | 10th day |
|---|---|---|
| Eucerin group | 0.22 ± 0.02 | 0.33 ± 0.02 |
| Persian oak ointment 1% | 0.23 ± 0.03 | 0.82 ± 0.05 |
| Persian oak ointment 2% | 0.25 ± 0.03 | 0.96 ± 0.02 |
| phenytoin-treated | 0.24 ± 0.03 | 0.86 ± 0.02 |
Histopathological examination promotion of wound healing by Persian oak ointment was confirmed by histological examination. Fibroblast cells, collagen fibers and blood vessels are prominently present in phenytoin and Persian oak ointment-treated groups as compared to the eucerin-treated group.
5. Discussion
In ethno-pharmacology facts, research on natural herbal products is planned on developing new drugs as a replacement for costly chemical drugs and their unwanted side effects. A number of studies have considered the advantages of plant products and their latent to become sympathetic therapy for wound healing due to absence of many surplus side effects and overall effectiveness (17). Research on the use of traditional drugs signified that as many as 80% people of the world still trust traditional herbal drugs to alleviate various skin diseases (8, 15). The aim of wound healing is to renovate the injured tissue to its normal structure as strictly as possible.
Skin is a complex tissue and consequently a full thickness wound results in damage to many organizations including: the basement membrane that underlies the epidermis, the epidermal keratinocyte layer and the dermis which is a complex structure that consists of extracellular matrix, nerves, fibroblasts, lymphatic vessels and blood. Additionally, a wound also causes hurt at the level of individual cells (17).
In an excisional model of wound, considerable wound healing activity was monitored in rats treated with ointment of Persian oak. A significant decrease in the period of epithelialization, superior scores of angiogenesis, and wound closure ratio was detected in these groups. Additionally, Persian oak ointment enhanced re-epithelialization and formulated new blood vessels. Shukla et al. (19), and Pattanayak and Sunita (12), proved the effects of some herbs (Dendophtthoe falcate, Centella asiatica, Aloe vera) on excisional wound healing in rats. Several plant foods such as Persian oak have water-soluble polyphenols such as tannins (commonly referred to as tannic acid). They have been reported to be guilty for decreases in feed efficiency, feed intake, net metabolizable energy, growth rate and protein digestibility in experimental animals. As a result, foods rich in tannins are believed to be of low nutritional worth. On the other hand, recent findings signify that the main effect of tannins was not due to their inhibition on food utilization or digestion but moderately the decreased efficiency in changing the absorbed nutrients to new body substances. Occurrences of toxicity have been reported to be related to the use of tannins-rich foods such as Persian oak recommending that tannins might be toxigenic but many tannin molecules and polyphenols have also been shown to diminish the mutagenic activity of a number of mutagens. Lots of mutagens produce oxygen-free radicals for interaction with cellular macromolecules. The antimutagenic potentials of tannins may be related to their antioxidative property, which is vital in protecting cellular oxidative damage. The generation of superoxide radicals was reported to be inhibited by tannins and related compounds. The antimicrobial activities of tannins are well-documented (10, 12, 15). The increase of various bacteria, fungi, viruses and yeasts was restrained by tannins. We have also found that propyl gallate, tannic acid and but not gallic acid, were inhibitory to off-flavor-producing microorganisms, aquatic bacteria, and foodborne bacteria. Antimicrobial properties of them appeared to be correlated with the hydrolysis of ester linkage between polyols hydrolyzed and gallic acid after ripening of many suitable for eating fruits. Tannins in these oaks thus supply as a natural protection mechanism against microbial infections. Oaks have also been reported to exercise other physiological effects, such as to reduce blood pressure and to hasten blood clotting. The kind of oaks and dosage are critical to these effects. Quercetin is a flavonoid found in many oak trees. Quercetin is used as anti-inflammatory and an antihistamine, as well as to treat heart conditions: quercetin has been demonstrated to reduce the risk of plaque buildup in the arteries that can result in heart attacks or strokes. As an anti-inflammatory, quercetin has redness-reducing, soothing properties. Flavonoids similar to quercetin are antioxidants, thus they help deactivate free radicals. Quercetin was a great proteasome activator and stimulated the growth of new cells. Moreover, it may help skin cells function better, reduce wrinkles and fine lines and develop the skin’s elasticity and texture (20).
Our results coincide with the text in terms of wound healing ratio, which was found to be higher than the studies formerly mentioned. Angiogenesis have an important role in wound healing and newly shaped blood vessels comprise 60% of the repair tissue. Neovascularization helps hypoxic wounds to attain the normoxic conditions (21, 22). Re-epithelialization plays a crucial role in cutaneous repair, depending upon the specific type of wound (4, 13, 17, 23). We detected that the Persian oak ointment increased the angiogenesis phase and re-epithelialization in addition to the general wound healing process. Specifically, the results of some studies assert that Persian oak ointment accelerates angiogenesis. Literature studies exhibit that most of the plant extracts useful in wound healing have antioxidant, antimicrobial, and anti-inflammatory effects (24, 25). The Persian oak plant extract had similar effects in previous studies (3, 5, 6, 10-13, 15, 19, 26). Moreover, we discovered that this herb contains significant amount of biologically active compounds (3, 5, 10, 27); therefore, the mechanism of wound healing may not solely be attributed to the antioxidant, antimicrobial, and anti-inflammatory properties of the herb. β-sitosterol, campesterol, and isofucosterol are some of the biologically active compounds of Persian oak. Navarro et al. (24) reported that campesterol, isofucosterol and β-sitosterol have anti-inflammatory effects through mechanisms such as neutrophil migration. Away from this effect, β-sitosterol enhances angiogenesis; thus, the authors consider that these biologically active compounds might increase Persian oak’s effects on wound healing. Based on the present consequences, the authors believe that Persian oak can be used to motivate wound healing or to efficiently treat wounds. Molecular oxygen plays a serious role in the pathogenesis and rehabilitation of chronic wounds. Overproduction of reactive oxygen species (ROS) results in oxidative stress in this manner causing cytotoxicity and delayed wound healing (28). Therefore, elimination of ROS could be an essential approach in healing of chronic wounds (28). A number of studies have explained that Persian oak is believed as a forceful antioxidant (29). Hence, Persian oak could also progress the wound healing based on its antioxidant properties. Nevertheless, more studies to reveal the exact mechanism of action of Persian oak ointment in wound healing will be required for verification. The aim of this assessment is to summarize and evaluate the vast and sometimes conflicting writing on oaks and to provide as precisely as possible the needed information for evaluation of the generally effects of oaks on human health.