1. Background
Free radicals and reactive oxygen species (ROS) are highly reactive molecules, formed in all tissues during normal cellular metabolism (1). However, different environmental factors, including tobacco smoke, pollutants, ionizing radiations, synthetic pesticides, and solvents are able to increase their production (2). ROS can react with biological molecules such as lipids, proteins and DNA. Oxidative damage caused by ROS, results in numerous pathological disturbances such as cancer, neurodegenerative, atherosclerotic, cardiovascular and inflammatory diseases (1). Human cells possess several antioxidant defense mechanisms to fight excess ROS induced oxidative damage; however, under stress condition dietary supplementation of synthetic antioxidants help the physiologic antioxidant defense system (2). Due to the toxic and mutagenic side effects of some synthetic antioxidant compounds such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT), great interest is recently focused on natural antioxidant (3). Redox properties of phenolic compounds are behind the main antioxidant potentials in herbs and species, which allow them to act as reducing agents, hydrogen donators and free radical quenchers (1). Many aromatic plants are effective in retarding the lipid peroxidation process in oils and fatty foods (3), which verify the use of essential oils as potential substitutes of the synthetic antioxidants (4, 5). The genus Artemisia L. is a large genera of the family Asteraceae or Compositae which mostly grows in Asia, Europe, and North America (6). Thirty four species of the Artemisia genus are found in Iran (7, 8). This genus contains chemical compositions such as monoterpenes, sesquiterpenes, sesquiterpene lactones, flavonoids, coumarins, sterols and polyacetylenes (9, 10). Different species of Artemisia have a vast range of biological effects including antimalarial (11), cytotoxic (12), antibacterial, antifungal and antioxidant activity (2, 9, 11). There is evidence for the antioxidant activity of various species of Artemisia (2, 13, 14). The antioxidant properties of the obtained extracts from these plants were also assessed in different areas (15-18). Artemisia dracunculus is one of the Artemisia members with numerous usage (19). Although there are some published reports on free radicals scavenging and antioxidant activity of A. dracunculus (20-22), there is no comprehensive study to evaluate antioxidant activity of various extracts as well as the essential oil of the plant.
2. Objectives
The current study performed the gas chromatography-mass-spectrometry (GC-MS) analysis of the essential oil isolated from the aerial parts of A. dracunculus for chemical composition elucidation. The current study aimed to evaluate the total phenolic, flavonoid and proanthocyanidin contents also the antioxidant activity of various extracts, essential oil and its main chemical composition.
3. Methods
3.1. Plant Materials
Artemisia dracunculus (tarragon) was collected from Pivehjan (Khorasan Razavi province, Northeastern of Iran) in July 2013. Voucher specimen (no. 10169) was identified by Mr. M.R. Joharchi affiliated to Ferdowsi university of Mashhad herbarium (FUMH) and deposited in the herbarium, department of pharmacognosy, Faculty of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran. All materials were stored at - 20°C (23).
3.2. Chemicals
All chemicals were obtained from Sigma (Sigma Aldrich GmbH. Sternheim), Merck (Darmstadt) and Roth (Karlsruhe) in Germany.
3.3. Extraction of Non-Volatile Components of the Sample
The shade dried aerial parts of A. dracunculus were cut in small pieces (500 g) and powdered by a blender and then macerated in pure methanol for 24 hours. The sample was then extracted by a percolator. The mixture was then concentrated at 50°C for dryness under reduced pressure (24, 25).
The dry extract was distributed in a minimum volume of methanol and water mixture (95:5). The resulted sample was fractioned three times with n-hexane to separate the non-polar compounds such as lipids. This defatting process was followed by partitioning of aqueous layer between dichloromethane (CH2Cl2), ethyl acetate (EtOAc) and n-butanol (n-BuOH) (26). The extracted solutions were concentrated at 50°C for dryness under reduced pressure. The aqueous fraction was freeze dried. All the extracted samples were stored at -20°C for further analysis.
3.4. Isolation and Identification of the Essential Oils
The volatile oil was isolated by steam distillation, using a Clevenger apparatus. Distillation continued for about four hours (27). The essential oil was dried by anhydrous sodium sulfate. The oil was kept in a sealed vial at -20°C for further analysis.
3.5. Chromatography-Mass Spectrometry and Gas Chromatography Analysis
The volatile oil composition of the aerial parts of A. dracunculus sample was analyzed by (GC-MS) and gas chromatography (GC). The GC-MS apparatus was an Agilent 5973, GC-MS spectrometer equipped with a fused-silica column (HP-5MS, 30 m × 0.25 mm i.d., film thickness 0.25 µm). The operating conditions were: oven temperature 50 - 300°C, after five minutes at 50°C the temperature increased to 240°C with the rate of 3°C/minute; the temperature increasing rate was 15°C/minute ranging 240°C - 300°C. The oven temperature remained at 300°C for three minutes; injector temperature 290°C; injector mode: split injection with a split ratio of 1:20; with the carrier gas, He; flow rate 2 mL/minute; electronic impact (EI), ionization potential 70 eV, ion source temperature 250°C, ionization current 1000 µA, resolution 1000 and mass range 40 - 300 u. The GC was an Agilent 6890, equipped with a FID detector. The chromatographic conditions were the same as in GC-MS analysis except for carrier gas, the nitrogen gas. The oil components were identified based on a comparison between their retention indices and those of analytical standards of available compounds, and matching mass spectral data of the oil constituents with those of Wiley7n library of MS spectra (27). Quantification of the relative amount of the individual components was performed according to the area percentage method without considering the calibration factor.
3.6. Total Phenolic Compounds Determination
Total phenolic contents of methanol, n-butanol, water, ethyl acetate and dichloromethane extracts were determined using the Folin-Ciocalteu method. Different concentrations of tannic acid (2 - 10 µg/mL) were used to plot a standard curve. Then 100 µL of each sample and standard was diluted with distilled water to the final volume of 0.5 mL. To each tube, 0.25 mL of the Folin-Ciocalteu reagent and 1.25 mL of the Na2CO3 solution (20%) were added. After the tubes vortexed and waiting for a 40 minute interval, the absorbance of all the samples and standards were measured at 725 nm using a UV-vis spectrophotometer (Cecil, UK). Using the plotted calibration curve, the total phenolic content of the samples was calculated and presented as tannic acid equivalent (TAE) (28, 29). All determinations were carried out in triplicates.
3.7. Flavonoids Content Estimation
Total flavonoid contents in each extracted sample were measured based on the formation of a flavonoid-aluminum complex. The flavonoids content was expressed as quercetin equivalents (QE) (30). Various concentrations (10 - 100 µg/mL) of quercetin (Sigma, USA) were used to construct a calibration curve.
Diluted samples (1 mL) were mixed with methanolic solution of aluminum chloride (AlCl3) (2%, 1 mL). After incubation of the samples for 15 minutes at room temperature, the absorbance of the reaction mixture was measured at 430 nm. All determinations were carried out in triplicates.
3.8. Proanthocyanidin Content Estimation
Determination of proanthocyanidin was based on acid hydrolysis and color formation. Different concentrations of catechin hydrochloride (Sigma, USA) were used to make the calibration curve. In brief, 0.5 mL of HCl was added to each diluted sample and to the standard samples containing various concentrations of catechin in methanol (0.0625 - 1 mg/mL) and left for 15 minutes at room temperature. The absorbance of all reaction mixtures was measured at 500 nm. The proanthocyanidin content was expressed as catechin equivalents (CE) (31). All determinations were carried out in triplicates.
3.9. Antioxidant Activity
3.9.1. DPPH Free Radical Scavenging Assay
In the DPPH (2, 2-diphenyl-1-picrylhydrazyl) assay, based on the scavenging ability of stable radical DPPH, fresh methanolic solution of DPPH (1 mL) was added to 2 mL of each of the methanol extract solutions with a concentration range of 2.5 - 100 µg/mL. The final DPPH was 0.1 mM. The mixture was shaken and incubated for 30 minutes at room temperature in the dark and then absorbance was measured at 517 nm using a UV-vis spectrophotometer (16). Methanol was used as a blank and ascorbic acid and butylated hydroxy toluene (BHT) (Sigma, USA) as positive controls. All tests were performed in triplicate.
The activity was calculated based on the following equation:
% Inhibition = 100 × (A0-A) / A0
where A0 was the absorbance of the control reaction (containing no test compound) and A was the absorbance of the test compound. IC50 values (inhibitory concentration 50 µg/ml) were calculated using the CALCUSYN program (16, 32).
3.9.2. Reducing Power Assay
In this method to measure the reducing power of the extracts, 2.5 mL phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of 1% potassium ferricyanide [K3Fe (CN)6] solution was added to 1 mL of extract samples with various concentrations. After mixing and incubating the mixture at 50°C for 20 minutes, the reaction was stopped by adding 2.5 mL of trichloroacetic acid (10%) solution to each sample. The samples were mixed and then centrifuged for 10 minutes. The supernatant (2.5 mL) was mixed with distilled water (2.5 mL) and 2.5 mL of FeCl3 (0.1% w/v) for 10 minutes. The absorbance at 700 nm was measured in a spectrophotometer as the reducing power. The higher absorbance of the reaction mixture indicated the increased reducing power. Ascorbic acid and butylated hydroxy toluene (BHT) were used as positive controls. All tests were performed in triplicate (1, 28).
3.9.3. Ferrous Chelating Activity Assay
In this method, the chelating activity of various extracts of A. dracunculus for ferrous ion was measured. To various concentrations of extracts (0.5 mL) deionized water (1.6 mL) and 0.05 mL of FeCl2 (2 mM) were added and mixed. It was followed by adding 0.1 mL of ferrozine (5 mM). Ferrozine reacts with the divalent iron to form stable magenta complex species. After incubating the samples for 10 minutes at room temperature, the absorbance level of the reaction mixtures was measured at 562 nm. The chelating activity (%) of the ferrous ion extract was calculated based on the equation given for DPPH scavenging assay. Methanol was used as blank and quercetin and EDTA were used as positive controls. All tests were carried out in triplicate (1, 28).
3.9.4. Trolox Equivalent Antioxidant Capacity Assay
In this measurement, the ABTS [2, 2’-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid)] / MnO2 method was used to evaluate the strength of the plant extracts to scavenge the preformed radical cation ABTS+. The reaction between ABTS (0.5 mM) and activated MnO2 (l mM) in phosphate buffer (0.l M) produces the ABTS+ radicals. Different concentrations of plant extracts (0.5 mL) were mixed with ABTS+ solution (3 mL) and the decay in absorbance was followed for 15 minutes at 734 nm. TEAC values were expressed in µM trolox equivalent/g of plant extracts (33). All tests were carried out in triplicate.
3.9.5. Thiobarbituric Acid Reactive Species Assay
To measure the potential antioxidant capacity of essential oil of the modified TBARS assay, egg yolk homogenates were used as lipid rich media, (3, 4). Briefly, 0.5 mL (10% w/v) of tissue homogenates and 0.1 mL of sample solution (oil concentrations: 0.1, 0.5, 1, 4, 20 and 40 g/L) freshly prepared in methanol before use, were added to a test tube and made up to 1.0 mL with distilled water. To induce lipid peroxidation, 0.05 mL of 2, 2’-azobis (2-amidinopropane) dihydrochloride (Aldrich, Germany) aqueous solution (0.07 M) was added to each sample followed by addition of 1.5 mL of 20% acetic acid (pH 3.5), and 1.5 mL of 0.8% (w/v) thiobarbituric acid in 1.1% (w/v) sodium dodecyl sulfate solution. The resulting mixture was vortexed and incubated at 95°C for 60 minutes. After cooling, 5.0 mL of n-butanol was added to each tube, then the content was extensively vortexed and centrifuged for 10 minutes. The organic upper layer was separated and the absorbance of the separated solution was measured using a spectrophotometer at 532 nm. Butan-1-ol was used as blank and vitamin E (α-tocopherol) and butylated hydroxy toluene (BHT) as positive controls. All tests were carried out in triplicate. Values were calculated based on the percentage of the antioxidant index (AI%) as follows:
AI% = 100 × (1- AT / AC)
Where AC was the absorbance value of the fully oxidized control and AT was the absorbance of the test samples.
3.9.6. Antioxidant Activity Measurement with the β-Carotene Bleaching (BCB) Test
A modified version of the β-carotene bleaching method was employed to measure the antioxidant activity of the volatile compounds. A solution of β-carotene (0.1 mg), linoliec acid (20 mg) and Tween 40 (100 mg) in chloroform were put together in a boiling flask and evaporation to dryness, under the vacuum at 50°C. By adding oxygenated distilled water (50 mL) and sonication for l minute; the mixture was emulsified to form an emulsion (A). Different concentrations (0.1, 0.5, 1.0, 2.0, 3.0 and 4.0 g/L) of ethanol stock solution (200 µL) of each antioxidant sample was mixed with 5 mL of emulsion (A) in a tube. A control containing no antioxidant, consisting of 200 µL of ethanol and 5 mL of emulsion (A) was also prepared. In another attempt, a second emulsion (B) consisting of 20 mg of linoleic acid, 100 mg of Tween 40 and 50 mL of oxygenated water was also prepared. A mixture of ethanol (200 µL) and 5 mL of emulsion (B) was used to reset the spectrophotometer. The absorbance level of all the samples was measured at 470 nm at time zero and then every 15 minutes for 120 minutes. BHT was used as positive control and all determinations were performed in triplicate (3). The percentage of inhibition was calculated based on the following formula:
%Inhibition = [(AA (120) - AC (120)) / (AC (0) -AC (120))] × 100
Where AC (120) was the absorbance value of the antioxidant at t = 120 minutes, AA (120) was the absorbance of the sample at t = 120 minutes and AC (0) was the absorbance of the control at t = 0 minute.
3.10. Statistical Analysis
Statistical analysis was performed using INSTAT 3 and prism 5 software. Data were analyzed using one-way analysis of variance followed by Tukey-Kramer post-hoc test for comparison between the groups. The level of significance was P < 0.05 (2).
4. Results
4.1. Total Phenolic, Flavonoid and Proanthocyanidin Contents
Total phenolic, flavonoid and proanthocyanidin contents of different extracts are presented in Table 1.
| Plant Extract | Total Phenolicsa | Total Flavonoidb | Total Proanthocyanidinsc |
|---|---|---|---|
| Methanol | 39.53 ± 0.74 | 13.28 ± 0.10 | 34.61 ± 0.39 |
| n-butanol | 12.82 ± 0.14 | 4.08 ± 0.03 | 4.35 ± 0.17 |
| Water | 9.30 ± 0.11 | 1.62 ± 0.06 | 8.60 ± 0.13 |
| Dichloromethane | 3.97 ± 0.01 | 0.55 ± 0.01 | 10.48 ± 0.05 |
| Ethyl acetate | 0.94 ± 0.01 | 0.34 ± 0.01 | 1.36 ± 0.01 |
| n-hexane | - | - | 16.17 ± 0.09 |
aResults expressed in mg tannic acid equivalent (TAE) / g dry aerial parts of A. dracunculus.
bResults expressed in mg quercetin equivalents (QE) / g dry aerial parts of A. dracunculus.
cResults expressed in mg catechin equivalents (CE) / g dry aerial parts of A. dracunculus.
4.2. Determination of Antioxidant Activity of the Extract
4.2.1. The Scavenging Activity of DPPH Radicals
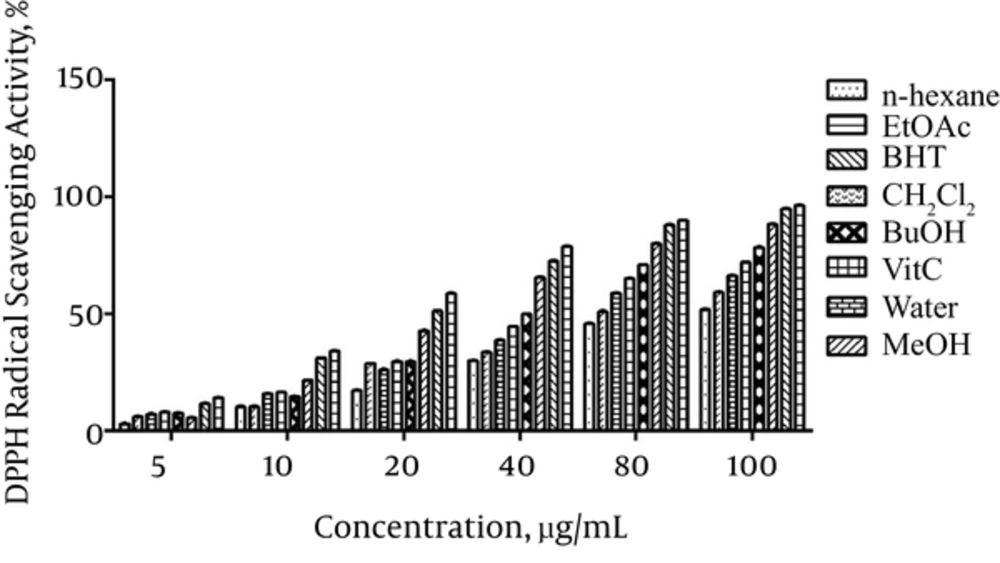
In DPPH test the antioxidant activity of the extracts was measured in terms of hydrogen donating or radical scavenging ability. The results presented in Figure 1 showed that DPPH radical scavenging activity of different extracts dose dependently increased in the order of vitamin C > BHT > methanol > n-butanol > ethyl acetate > water > dichloromethane > n-hexane.
4.2.2. Reducing Power Assay
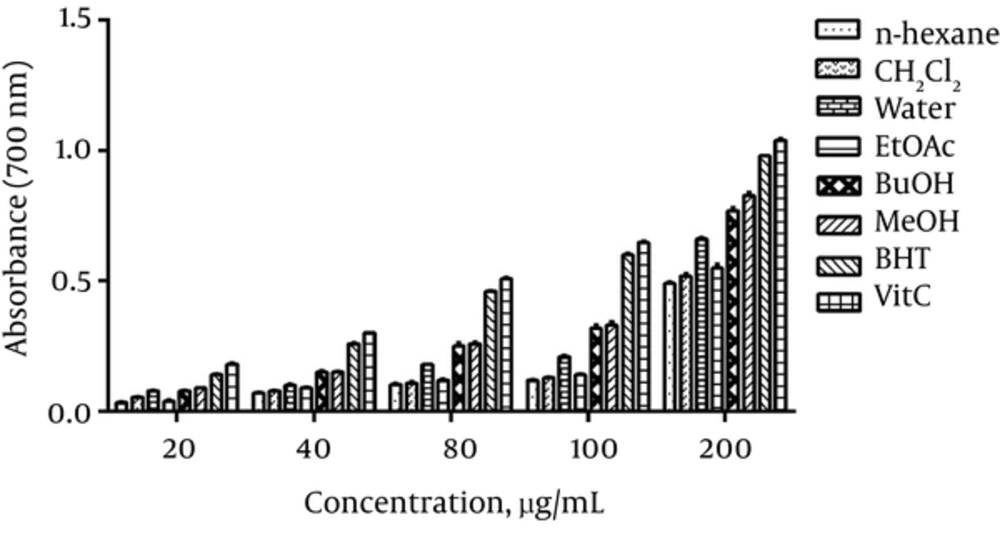
As shown in Figure 2, the reducing power of various extracts increased in a dose dependent manner. Methanol extract showed the highest reducing power and n-hexane extract showed the lowest power among other extracts.
4.2.3. Ferrous Ions Chelating Ability
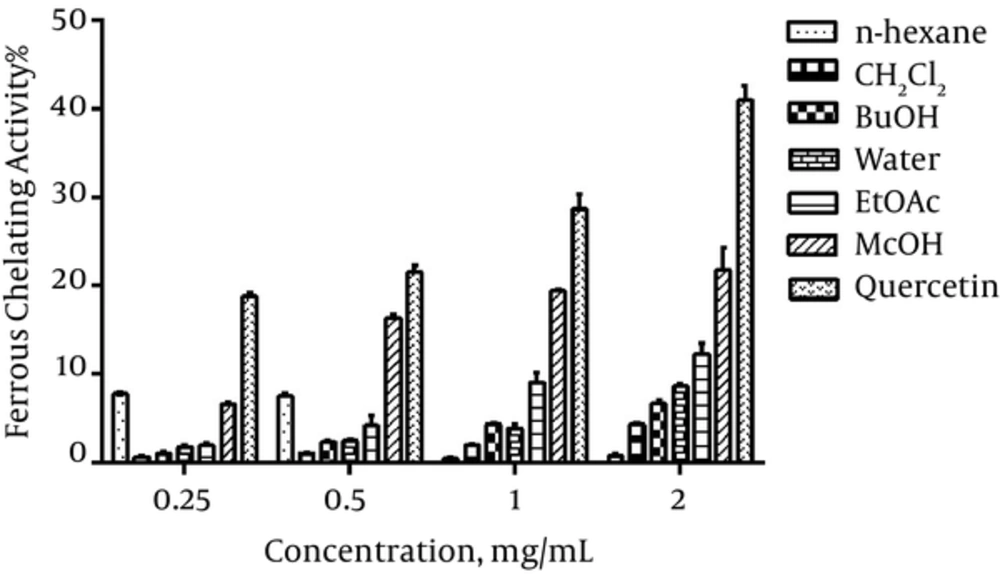
The ferrous ions chelating ability of different extracts increased dose dependently and methanol extract showed the highest activity compared to others (Figure 3).
4.2.4. Trolox Equivalent Antioxidant Capacity (TEAC Assay)
As presented in Table 2, radical ABTS+ ability of various extracts (at concentration of 20 µg/mL) was as follows: methanol > water > n-butanol > n-hexane > ethyl acetate > dichloromethane extract.
| Sample or Extracts | Trolox Equivalent Antioxidant Capacity | ||||
|---|---|---|---|---|---|
| 20b | 10 | 5 | 2.5 | 1.25 | |
| Methanol | 363.61 ± 3.83 | 337.67 ± 13.09 | 476.31 ± 21.24 | 887.97 ± 36.80 | 666.83 ± 104.75 |
| n-hexane | 15.20 ± 0.48 | 21.54 ± 0.91 | 32.24 ± 1.15 | 48.59 ± 7.16 | 59.90 ± 9.23 |
| Dichloromethane | 9.95 ± 0.32 | 11.89 ± 0.64 | 18.54 ± 0.80 | 27.70 ± 2.80 | 27.90 ± 17.49 |
| Ethyl acetate | 10.36 ± 0.07 | 11.89 ± 0.34 | 11.14 ± 0.60 | 8.71 ± 1.30 | 3.67 ± 1.27 |
| n-butanol | 54.06 ± 1.02 | 55.94 ± 2.82 | 41.81 ± 4.26 | 30.08 ± 18.95 | - |
| Water | 99.46 ± 1.25 | 23.24 ± 7.2 | 14.60 ± 6.90 | - | - |
aTEAC value was expressed in µM trolox equivalent/g for the plant extracts.
bConcentrations (µg/mL).
4.3. Chemical Composition of Artemisia dacunculus Essential Oil
The components of the volatile oil sample extracted from the aerial parts of A. dracunculus were identified using GC and GC/MS analyses. The oil was clear and possessed a strong odor and yielded 0.4% (v/w). The constituents of the essential oil and their relative amount were listed in order of elution from the DB5 column. Thirty three compounds representing 99.91% of the essential oil compounds were identified (Table 3). The major components in the essential oil were oxygenated monoterpene hydrocarbons. The main oxygenated monoterpenes of the plant’s oil were estragole (57.1%) and methyl eugenol (9.5%). The two sesquiterpene hydrocarbons, Germacrene-D (7.1%) and bicyclogermacrene (5.2) were the other major components of the A. dracunculus volatile oil.
| Component | Retention Indicesb | Percentage |
|---|---|---|
| 2-hexanal | 857 | 0.1 |
| Myrcene | 993 | 0.1 |
| α-phellandrene | 1007 | 0.2 |
| p-cymene | 1023 | 0.1 |
| Limonene | 1032 | 0.3 |
| (Z)-β-ocimene | 1041 | 1.3 |
| (E)-β-ocimene | 1053 | 0.5 |
| Terpinolene | 1086 | 1.9 |
| Linalool | 1099 | 0.2 |
| allo-ocimene | 1129 | 0.1 |
| Estragolec | 1200 | 57.1 |
| Carvone | 1241 | 1.0 |
| Piperitone | 1258 | 0.1 |
| δ-elemene | 1334 | 1.4 |
| α-copaene | 1379 | 0.1 |
| β-elemene | 1387 | 0.2 |
| Methyl eugenolc | 1408 | 9.5 |
| β-caryophyllene | 1421 | 1.6 |
| α-humulene | 1452 | 0.8 |
| (E)-β-farnesene | 1460 | 3.3 |
| Germacrene Dc | 1487 | 7.1 |
| Bicyclogermacrenec | 1496 | 5.2 |
| (E, E)-α-farnesene | 1509 | 0.6 |
| γ-cadinene | 1515 | 0.2 |
| δ-cadinene | 1525 | 0.3 |
| Elmicin | 1555 | 0.9 |
| Para-methoxy cinnamaldehyde | 1566 | 0.3 |
| Spatholenol | 1582 | 4.5 |
| Hexadecane | 1596 | 0.1 |
| Cedrol | 1604 | 0.1 |
| Tau-muurolol | 1644 | 0.2 |
| α-cadinol | 1655 | 0.4 |
| Heptadecane | 1698 | t* |
| Grouped compounds | ||
| Phenyl propanoeids | 67.6 | |
| Monoterpene hydrocarbons | 4.5 | |
| Oxygenated monoterpens | 1.6 | |
| Sesquiterpene hydrocarbons | 20.8 | |
| Oxygenated sesquiterpens | 5.2 | |
| Miscellaneous | 0.2 | |
| Total | 99.9 |
at: trace < 0.05%.
bRI: The Kovats retention indices relative to C8 - C20 n-alkanes were determined on HP5 capillary column.
cThe structure of the major oil components (over than 5%) were being approved with mass and retention indices were published in www.webbook.nist.gov (Ref. (34) in addition to identification procedure that mentioned in experimental method.
4.4. Determination of Antioxidant Activity of Artemisia dracunculus Essential Oil
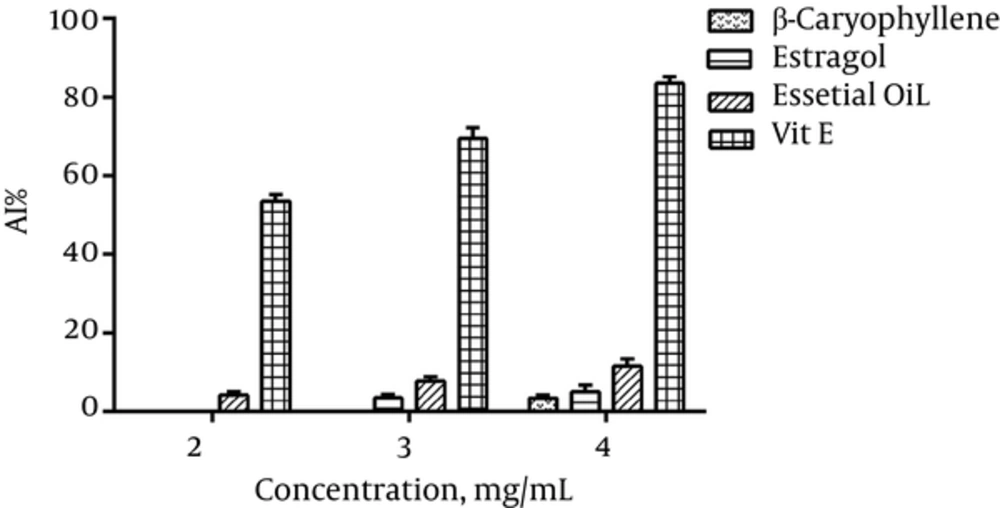
4.4.1. Thiobarbituric Acid Reactive Species Assay
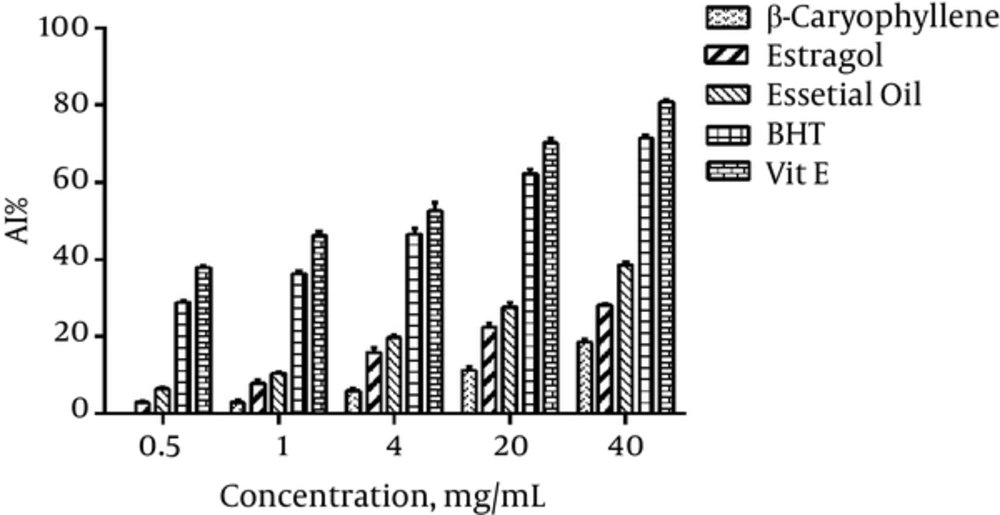
As compared to the Vit E and BHT, A. dracunculus essential oil and its components showed significant difference (Figure 4).
4.4.2. Antioxidant Activity Measurement with the β-Carotene Bleaching Test
Artemisia dracunculus essential oil and its components showed weak antioxidant activity when compared to BHT (Figure 5).
A significant difference was observed in antioxidant activity of different extracts as well as the essential oil and its components when compared to the positive controls (P < 0.001).
5. Discussion
Natural products have been used for a long time to prevent and treat many diseases. Therefore, there are considerable interests in elucidating the mechanisms of their actions (1). It is stated that the various Artemisia species have a wide range of biological effects (2, 9, 11, 12). Recently, some studies reported antioxidant activity of waster extracts and the essential oil of A. dracunculus (20, 21, 35). Since there was no comprehensive study on evaluating the antioxidant capacity of various organic and aqueous extracts as well as essential oil of A. dracunculus, the current study evaluated the antioxidant activity of A. dracunculus using different methods such as DPPH, reducing power, ferrous ions chelating, TEAC, TBARS and BCB assay.
As presented in Table 1, the amounts of phenolic compounds varied widely and ranged from the highest level of 39.53 ± 0.74 mg TAE/g of dry aerial parts of A. dracunculus in the methanol extract to the lowest level of 0.94 ± 0.01 in ethyl acetate extract. Flavonoid contents ranged from 13.28 ± 0.10 to 0.34 ± 0.01 mg QE/g of dry aerial parts of A. dracunculus. The maximum level was observed in methanol extract and the lowest level in ethyl acetate extract. The proanthocyanidins contents were 34.61 ± 0.39 mg catechin equivalents (CE)/g of dry aerial parts of A. dracunculus, the highest level in the methanol extract to 1.36 ± 0.01 and the lowest level in ethyl acetate extract. Since solvents with different polarities can extract compounds with various polarities the range of total phenolic, flavonoid and proanthocyanidin contents is different in samples (36).
Most of the antioxidant potentials in herbs and species are due to the redox properties of phenolic compounds that allow them to act as reducing agents, hydrogen donators and free radical quenchers (4, 37).
Due to the complex nature of phytochemicals, the antioxidant activity of plant extracts cannot be evaluated only by a single method; therefore, it is important to employ several accepted antioxidant assays to assess the antioxidant activity of plant extracts (1, 28, 29, 36). In the present investigation, some of the most commonly accepted assays were used to explain the function of antioxidants.
The total phenolic contents have direct correlation with DPPH radical activity (15, 38). The highest radical scavenging activity was shown by methanol extract with the lowest IC50 value of 26.6 mg/L in comparison to the IC50 value measured for vitamin C (16.1 mg/L). The reducing power is one of the important antioxidant methods to evaluate the antioxidant capability of medicinal herbs (16).
Polyphenols can chelate pro-oxidant metal ions, thus, preventing free radical formation from these pro-oxidants (16). In the presence of chelating agent in the sample, the complex formation (Fe2+-ferrozine) is disrupted, resulting in decrease of red colored complex (28). Although the various concentrations of different extracts had a weak ability of chelating ferrous ion, the methanol extract (in a concentration of 2 mg/mL) showed the highest chelating activity.
TEAC assay is an excellent method to determine the antioxidant activity of a broad diversity of substances, such as hydrogen-donating antioxidant or scavenger of aqueous phase radicals or scavengers of lipid peroxyl radicals (39). The results obtained from TEAC assay demonstrated that extracts with high content of phenolics are considered as potent donors of hydrogen (33).
The essential oil components are susceptible to the growing environment of the plant; therefore, it is expected to have wide variations in the composition of essential oils extracted from the plant grown in different places as it is for tarragon (35). Estragole (57.2) and methyl eugenol (9.5) were the two main components of the identified oil using GC and GC/MS analyses.
The evaluation of radical scavenging activity of the essential oil using TBARS assay showed a moderate antioxidant activity of this oil compared with vitamin E activity. The amount of AI obtained from the A. dracunculus essential oil using BCB test showed a very low antioxidant activity of this oil.
In conclusion, the variety of antioxidant activity results obtained from various extracts (methanol, dichloromethane, water, n-hexane, ethyl acetate and n-butanol extract) indicated the presence of phytochemicals from different classes of structure. However, the methanol extract showed the highest activity in all tests when compared with the activity of the controls. The methanol extract was a rich source of antioxidants, with a significant high level of phenolic, flavonoid and proanthocyanidin content. The antioxidant activity of the essential oil had higher activity in TBARS method in comparison with BCB test. These differences showed that anti-oxidative mechanisms responsible for the antioxidant effects of this plant are probably more similar to those of TBARS assay rather than other tested methods.
To characterize the active ingredients, further phytochemical studies are needed. In addition, there is a need to use even more antioxidant activity methods to assess their antioxidant activity.