1. Background
Pulmonary fibrosis (PF) is a complex, chronic condition that is generally considered to be the result of a variety of acute and chronic lung diseases. PF is characterized by increasing collagen content, inflammation, excessive matrix deposition, and destruction of the lung architecture, ultimately leading to respiratory failure (1).
Although some of the etiological aspects of PF remain unknown, cigarette smoking, viral infections, and surfactant protein polymorphisms have been proposed as risk factors for the progression of PF (2). To date, no standard treatments have been introduced in clinical settings, although glucocorticoids and cytotoxic drugs have been utilized (3). Death usually occurs as the result of respiratory complications; the survival rate is roughly 50 percent within five years (4, 5). Angiotensin-converting enzyme (ACE), via proteolytic actions on angiotensin I, produce angiotensin II. Angiotensin II (Ang II), as a bioactive peptide of the renin-angiotensin system (RAS), plays an important role in the initiation and maintenance of lung fibrosis (6). A growing body of evidence indicates that Ang II regulates the fibrotic response to tissue injury via the induction of the proliferation of human lung fibroblast, the production of lung procollagen (7-9), and the induction of alveolar epithelial cell apoptosis by the binding of Ang II to the AT1 receptor (8, 10). Studies have also shown that angiotensin-converting enzyme inhibitors (ACEIs) (11) and Ang II type 1 receptor blockers (ARBs) (12) may be used for the management of PF in animal models and humans. Related studies in the literature have primarily concentrated on discovering the associated mechanisms through which these medications improve PF pathological indices.
In the current assessment, we chose to examine an angiotensin receptor blocker known as valsartan, which has been applied for several years in different clinical conditions, including controlling hypertension and heart failure (13), utilizing valsartan’s presumptive actions in blocking the binding of Ang II to the Ang II type 1 receptor (AT1).
2. Objectives
The present research specified the anti-fibrotic and probable protective effects of valsartan as an angiotensin receptor antagonist for investigation; future cellular studies and other experiments will be required to determine the exact nature of valsartan’s beneficial involvement in this process.
3. Methods
3.1. Animals
Healthy male Sprague-Dawley (SD) rats weighing 200-220 g were purchased from the animal house and research center of Jundishapur University of Medical Sciences, Ahvaz, Iran. Animals were kept at 23 ± 2°C with an alternating cycle of 12 h light and dark. The rats had free access to food and tap water ad libitum. The animal experimental protocol used was according to the Jundishapur University Animal Care and Use Committee.
3.2. Drug Treatment Groups
On the basis of the primary evaluations and previous studies, the optimal doses of valsartan were selected (20, 40, and 80 mg/kg). The animals were randomly divided into five groups of eight rats each, as follows. The control group received a single intratracheal (IT) dose of bleomycin (BLM) solution (7.5 IU/kg), while the vehicle group was given a single PO dose of saline (0.2 ml/kg). Each treatment group received valsartan (Dr. Abidi Pharmaceutical Company, Iran) in doses of 20, 40, or 80 mg/kg, seven days before administration of a single IT dose of BLM (7.5 IU/kg); treatments were continued for up to three weeks. The day for BLM (IT) injection was designated as the seventh day. Valsartan and saline were given once daily in the treatment and vehicle groups (respectively) for four weeks.
3.3. Induction of Pulmonary Fibrosis by Bleomycin
According to Schraufnagel et al.’s method (14), on the seventh day of treatment, the rats were anaesthetized with ketamine (50 mg/kg) intraperitoneally (IP), placed on a slanted board, and hung from their upper incisors. Keeping the nose of each animal closed and its tongue out, BLM solution (7.5 IU/kg) was administrated into the trachea via the mouth by a modified needle. After recovery from anesthesia, the rats were carefully returned to their cages.
3.4. Isolation of Lung Tissue
At the end of the treatment course, all rats were weighed. The animals were sacrificed, and their lungs were dissected out; the whole lung weight was then recorded. One part of each lung was fixed with 10 percent buffered formalin for histopathological evaluation; another part of each lung was separated for measurement of hydroxyproline. Lung specimens were stored at -70°C until hedroxyproline assay could be conducted.
3.5. Determination of Lung Index
Each rat was weighed before sacrifice. The lungs were removed and weighed using an electronic balance; the lung index was expressed as the ratio of lung wet weight (mg) to body weight (g).
3.6. Histological Evaluation
Sections of fixed embedded tissues were cut to 4 μm using a rotary microtome, and then the lung tissue sections were stained with hematoxylin and eosin (H&E). Finally, all tissue sections were studied with light microscopy.
3.7. Hydroxyproline Measurement in Lung Tissue
Hydroxyproline is considered to be a good index of collagen deposition. Lung samples were homogenized with homogenizer and then hydrolyzed in 6 N HCl for 18 hours at 110°C; 2.5 M NaOH was then added to neutralize the samples. Chloramine T (1 mL), perchloric acid (1 mL), and dimethylaminobenzaldehyde (1 mL) were added to the samples. The absorbance of the samples were read to be 550 nm in a spectrophotometer. The results were expressed as milligrams of hydroxyproline (HP) per gram of lung tissue (15).
3.8. Statistical Analysis
The data are presented as the mean +/– scanning electron microscope (SEM) values. Statistically significant differences between groups were determined by ANOVA followed by Tukey’s test. Significance was accepted when P < 0.05.
4. Results
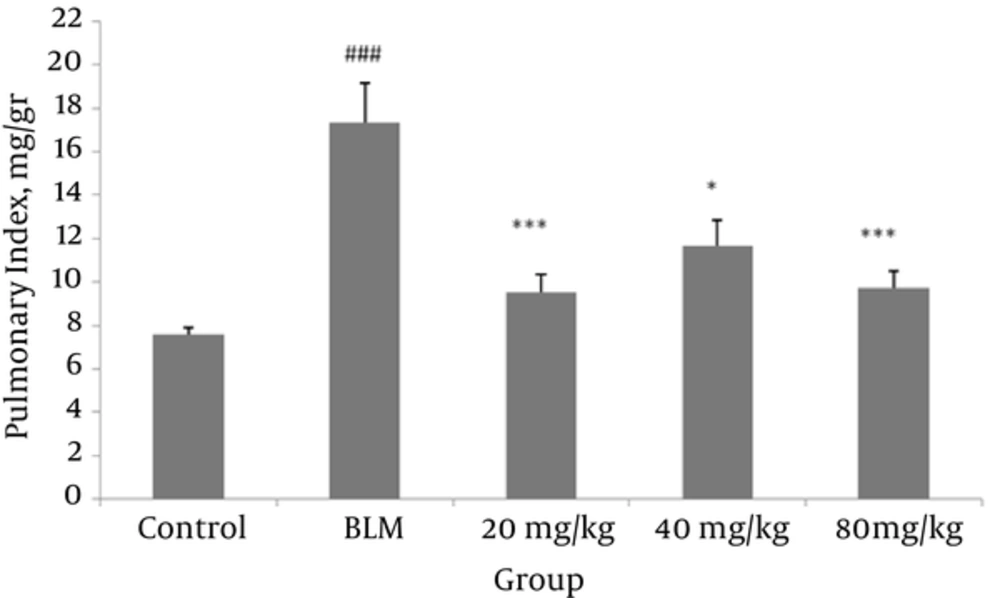
4.1. Effect of Valsartan on the Lung Index
An increase in the lung index is one of the indices representing lung inflammation. As shown in Figure 1, lung index values decreased significantly in comparison to the BLM group; the lung index values significantly increased in the BLM-induced PF model in comparison to the control group.
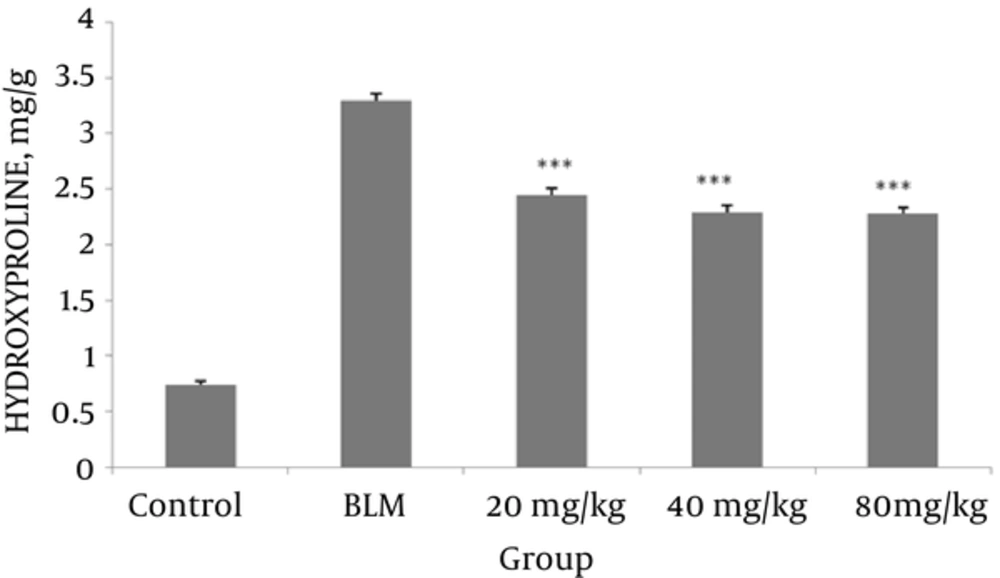
4.2. Effect of Valsartan on Hydroxyproline Content
As shown in Figure 2, the hydroxyproline content in the lung tissues was significantly increased in the BLM-exposed groups; valsartan in the three different doses (20, 40, 80 mg/kg) markedly decreased the hydroxyproline content in comparison to the BLM group.
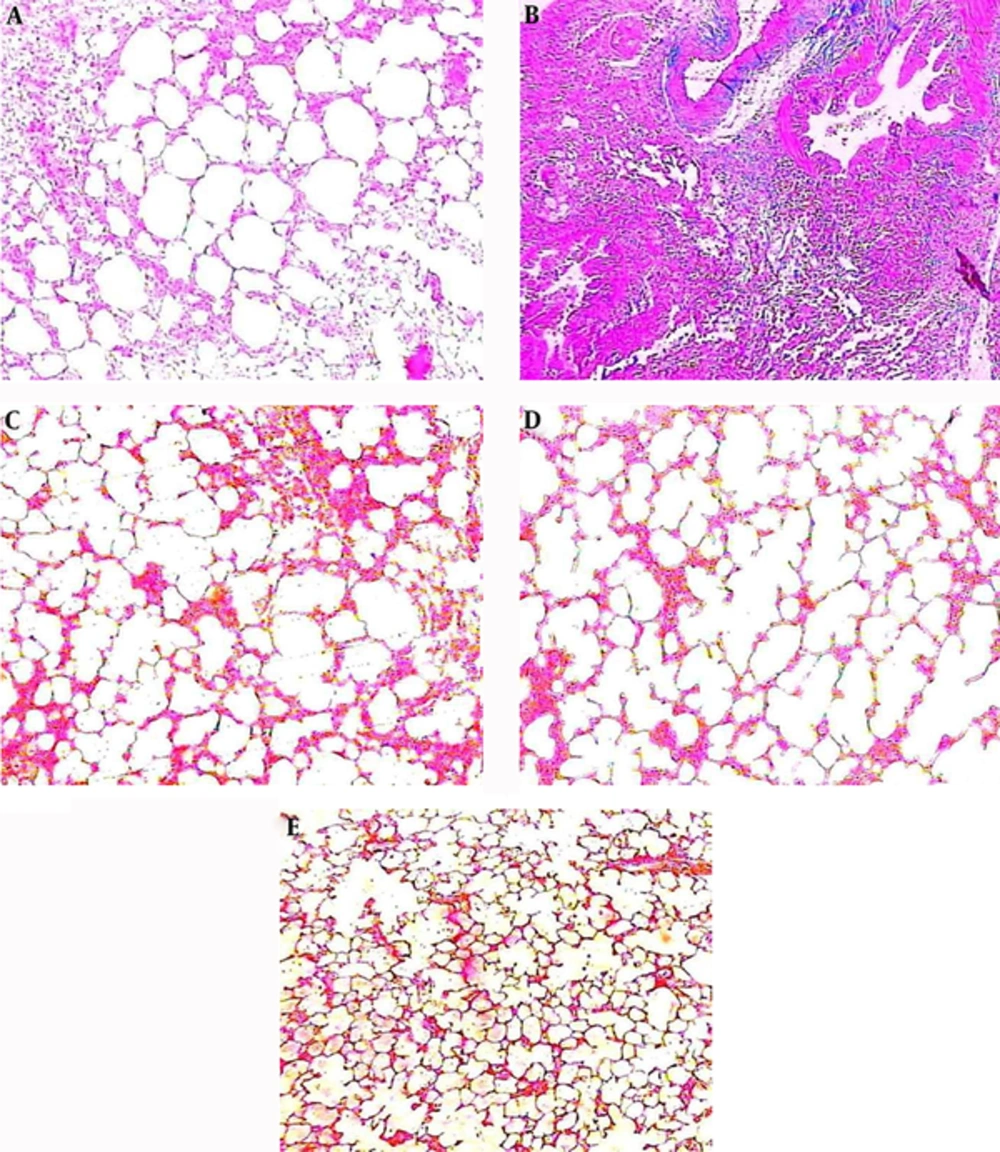
4.3. Effect of Valsartan on Alveolar Inflammation and Lung Fibrogenesis
The histopathological findings of the lung tissues showed that for the control group, alveolar spaces, alveolar septa, and lung structure were normal, and no lesions were evident (Figure 3A). The administration of bleomycin precipitated significant histological changes, including detectable areas of inflammatory infiltration, increases in the thickness of the alveolar walls, and collagen deposition and fibroblastic manifestations (Figure 3B). Adding valsartan in different doses showed significantly reductive changes in lung morphology: less inflammation, less cell infiltration, and less collagen deposition and septal widening were observed (Figure 3C, D, E). Pathological changes were observed, however, among the three doses of valsartan (20, 40, 80 mg/kg); the severity of the changes differed from slight to moderate.
5. Discussion
Pulmonary fibrosis is a progressive fatal lung disease (1). No satisfactory therapeutic agent has been found, and the current protocols have not indicated their expected efficacy nor their possibly severe adverse effects. A growing body of studies have thus been undertaken to find novel and effective ways to improve the management of the consequences of this fatal condition (5, 16). Most studies to date have used the bleomycin-induced pulmonary lesion as a model for fibrosis of lungs in rats (4, 5).
In this analysis, we evaluated the anti-fibrotic effects of valsartan as an angiotensin receptor antagonist in bleomycin-induced pulmonary fibrosis. The present study showed that valsartan significantly decreased lung inflammatory infiltration after bleomycin exposure. Several studies have reported an anti-inflammatory effect of AT1 receptor blockade (17-19). An enhancement in lung hydroxyproline content (as a remarkable indicator of collagen quantity) was observed on the 21st day of treatment after bleomycin exposure in the bleomycin group. In the treatment groups, the hydroxyprolin content was markedly decreased. Histopathological evaluation showed that valsartan noticeably reduced the extent and severity of the histological signs of tissue damage.
Corresponding in vitro studies have shown Ang II to be pro-fibrotic, thus causing an up-regulation of collagen gene expression in human lung fibroblasts (7). Ang II as a key mediator in the pathogenesis of lung fibrosis could influence the progression of lung injury via a number of mechanisms (17). Several previous investigations have reported an increase in lung Ang II concentrations and lung collagen content. Up-regulation in AT1 receptor expression occurred in lung parenchyma after bleomycin-induced lung injury. These studies’ results suggest that angiotensinogen and AT1 receptors are expressed in lung tissue (7, 8, 17). Previous assessments have also shown that AT1 receptor antagonists decrease lung collagen deposition, which was confirmed in our observations (7, 17, 20). Valsartan could attenuate pulmonary fibrosis in both the treatment and pre-treatment stages. Our histological evaluation of the lung tissue and the reduction of hydroxyproline content indicated a protective effect of valsartan.
Our findings are in agreement with those of Molina-Molina et al. (21), who showed that losartan attenuated pulmonary fibrosis by increasing prostaglandin E2. Otsuka et al.’s (17) studies on candesartan indicated that candesartan could decrease the concentration of neutrophils in bronchoalveolar lavage (BAL) fluid in the bleomycin exposure group, which suggests that Ang II may play an important role in neutrophilic infiltration in the lung. Long et al. (22) showed an anti-fibrotic effect of valsartan via increased collagen synthesis through the induction of connective tissue growth factor (CTGF) expression. Yao et al. investigated the anti-fibrotic effect of losartan and found that the protective effect of losartan may be associated with its anti-free radicals and the reduction in TGF-β1 (23). The results of Ghazi-Khansari et al.’s work (24) showed that captopril and enalapril improved pulmonary fibrosis in histopathological studies as well as decreasing collagen content in the lung tissue. Marut et al.’s research (25) on irbesartan as an AT1 blocker indicated a protective effect of irbesartan by the inhibition of inflammation and NO production.
The data gathered in the current assessment lend support to the protective effect of valsartan as an angiotensin receptor blocker and provide evidence that further work should be conducted on angiotensin receptor antagonists as a promising treatment option for pulmonary fibrosis. Molecular and genetic studies are necessary to clarify the exact involvement mechanisms of the anti-fibrotic influences of valsartan.