1. Background
Liver toxicity induced by CCl4 can cause liver injury through the formation of reactive free radicals that can bind covalently to cellular macromolecules forming nucleic acid, protein and lipid adducts. CCl4 can affect hepatocellular calcium homeostasis through the induction of hypomethylated ribosomal RNA, resulting in the inhibition of protein synthesis (1). Based on the conducted research, CCl4 leads to liver inflammation and fibrosis. The toxic effects of CCl4 result from the fact that its bioactivity with the help of the P450 cytochrome enzyme converts it into trichloromethyl free radicals (CCl3*) that are present in the first stage of CYP2E1 in the endoplasmic reticulum network. In the next stage, CCl3*, together with other molecules of its more active form, reacts to oxygen and produces the trichloromethylperoxy free radicals (CCl3OO*), following which a chain of chemical reactions starts that raises CCl4 toxicity to its highest level (2).
Liver toxicity is monitored in standard toxicity studies by many investigations, including clinical biochemistry parameters (enzymes, proteins, lipids, etc.) (3).
Liver fibrosis is a condition characterized by an increase in extracellular matrix proteins such as collagen type I and III, proteoglycans, and fibronectinand elastin. In normal conditions, matrix metalloproteinase (MMP) is made by stellate cells and adjusts the production of extracellular matrix. This condition usually starts with an inflammatory phase which, after chronic oxidative stress, eventually proceeds to fibrosis (4).
Lipid peroxidation is a term that usually refers to the oxidative decomposition of unsaturated fatty acids of the membrane phospholipids (5). This process is very common in pathological conditions, and it usually starts with the reaction of ROS (reactive oxygen species) and other free radicals with polyunsaturated fatty acids, which is magnified by the presence of divalent metal ions, and as a result final aldehyde products such as malondialdehyde (MDA) and other products are produced (6). Glutathione (GSH) can detoxify hydrogen peroxide and lipid peroxidation (7). A GSH level decrease along with fibrosis/cirrhosis pathological symptoms has been reported by a variety of stimuli, including CCl4, which is a widely used chemical for experimental induction of fatty liver and liver fibrosis in animals (8, 9).
Platelets contain not only proteins needed for homeostasis but also have many growth factors, including platelet-derived growth factor, transforming growth factor, vascular endothelial growth factor, insulin like growth factor, and epidermal growth factor. Previously, we demonstrated how to protect the liver from CCl4-induced damages and attenuated oxidative stress by an increase in GSH content of the liver tissue (10).
The ability of mesenchymal stem cells (MSCs) to be differentiated from other cell types makes them an attractive therapeutic tool for cell transplantation (11, 12). The in vitro hepatogenic differentiation of adult stem cells has been the subject of different reports. In a previous study, we have transdifferentiated human MSCs into liver cells, using insulin-like growth factor 1, which despite its important role in liver development, has not been used for in vitro hepatic differentiation (13). The immunomodulatory effect of MSCs makes them a promising candidate progenitor cell for regenerative medicine, especially in organ transplantation (14, 15). Therefore, in this study, we used human MSCs- xenotransplantation to examine these cells in an animal model, following our in vitro results.
Recently, the MSC has been studied with the prospect of treating acute and chronic liver diseases. Some studies provide clinical and experimental evidence suggesting that MSC transplantation can restore the function of the liver in conditions of acute and chronic damage (16), or evidence of antioxidant effect of these cells (17).
2. Objectives
The aim of this study was to compare the therapeutic effects of marrow-derived MSC and PE injection in rats with CCl4 induced liver toxicity.
3. Methods
3.1. Chemicals
CCl4, calcium gluconate, sodium dodecyl sulfate, ethylene diamine tetra acetic acid (EDTA), 5, 5’-dithiobis-(2-nitrobenzoic acid) or DTNB, Tris, thiobarbituric acid (TBA) and trichloroacetic acid were purchased from sigma chemical company, Germany. All other chemicals were of highest quality available in the market.
3.2. Isolation of Human MSCs (hMSCs)
Human MSCs were obtained from 5 mL bone marrow aspirates from iliac crest of normal donors within the age range of 19 - 45 years. They were donors of bone marrow to a related patient. After obtaining the approval of the ethic committee, written informed consent was also obtained to allow analyzing the clinical data and tests. Each sample of aspirate was diluted 1:1 with DMEM-low glucose (Invitrogen, Merelbeke, Belgium) and layered over about 5 mL of ficoll (Lymphoprep; Oslo, Norway). The isolation method was according to our previously reported method (13) by some modifications, which will be mentioned completely. The mononuclear cell layer was removed from the interface after centrifugation at 939 g for 20 minutes. Cells were suspended in DMEM and centrifuged at 338 g for 15 minutes, and were then suspended in basal DMEM medium containing 10% fetal calf serum (Invitrogen, Merelbeke, Belgium), 1% penicillin (Invitrogen, Merelbeke, Belgium), 1% streptomycin (Invitrogen, Merelbeke, Belgium), and 2 mM glutamine (Invitrogen, Merelbeke, Belgium). The cells were seeded at a density of 80.000/cm2 in 25 cm2 T-flasks and maintained at 37°C in an atmosphere of 5% CO2. After four days, the non-adherent cells were removed and the media was changed every three days. To expand MSCs, the adhered monolayer was detached with trypsin-EDTA (Invitrogen, Merelbeke, Belgium) at 37°C for five minutes, after 14 days for the first passage and every 4 - 5 days for successive passages. During in vitro passaging, the cells were seeded at a density of 5 - 10 × 103 cells/cm2 and expanded for several passages until they no longer reached confluence. Human MSCs (hMSCs) were isolated, using a method previously used (16). Cells from human bone marrow (BM) were taken from the posterior iliac crest bone by aspiration in Namazi Hospital (17).
3.3. Preparing Platelet Extract
Fourteen female rats (170 - 200 g) were selected from the laboratory animals research center in Shiraz University of Medical Sciences. The rats were anesthetized with ether, and their blood sample was collected via open chest cardiac puncture. Then, the blood was centrifuged in two steps by Lucarelli procedure. Platelets were counted, and autologous thrombin was prepared as Lucarelli et al.’s method (18). Then calcium gluconate was added, and platelet secretion was centrifuged at 1520 g for five minutes to reduce the presence of platelet membrane fragments. The supernatant was filtered with a 0.22 µm pore filter, divided into aliquots, and frozen at - 80°C for subsequent use (19). The protein concentration in the filtrates was determined by Bradford method (150 mg/mL) (20).
3.4. Preparing the Animals and the Experimental Groups
A total of 35 adult male Wistar rats weighing 250 - 300 g were purchased from Shiraz Razi institute for serum and vaccine and divided into three groups (7 rats in each group). Group I (negative control group) was the group of normal rats that did not receive any CCl4; they only received olive oil intraperitoneally (IP) (0.5 mL/kg) twice a week for eight weeks. The positive control group (Group II) received CCl4 diluted 1:1 in olive oil IP (1 mL/kg) twice a week for eight weeks. Group III, in addition to receiving CCl4 IP (1 mL/kg), received the prepared cells (1 × 106 cells/rat in 1 mL PBS) by insulin syringe into several lobes of the liver in a completely sterile environment in the fourth week of CCl4 injection (21). Group IV received PE (0.5 mL/kg 1:1 in PBS, s .c. n = 7) two days a week for three weeks; and group V received the CCl4 (1 mL/kg body weight as a 1:1 mixture with olive oil i.p.) twice a week for eight weeks. Five weeks after CCl4 injection, the rats received PE (0.5 mL/kg 1:1 in PBS s. c. n = 7) two days a week for three weeks. After the eighth weeks of CCl4 injection, the rats were anesthetized, using thiopental (50 mL/kg) and their blood samples were collected from the vena cava. Their blood was collected to provide serum, and their liver was dried after being washed with normal saline. Some parts of them were used to assess the oxidative stress, and the rest were fixed in formalin (10%). Histological sections (5 μm) of the liver-lobes were obtained, stained with Hematoxylin and Eosin and transported to the laboratory for histopathological examinations.
3.5. Histopathological Studies
A part of the liver was removed from the animals and the tissue was fixed in 10% formalin for at least 24 hours. Then, the paraffin sections were prepared (by Automatic tissue processor, Autotechnique) and cut into 5 µm thick sections by a rotary microtome. The sections were stained with Hematoxylin and Eosin dye, and were studied for histopathological changes; i.e., necrosis, fatty changes, ballooning degeneration, and inflammation. Histological damages were scored as follows: 0: Absent; +: Mild; ++: Moderate; +++: Severe.
3.6. Immunohistochemistry Analysis
We quantified the proliferation of hepatic myofibroblasts in the liver tissue sections, using double immunostaining with antibody to α-SMA (a marker of fibrosis) and Ki-67 (a marker of liver regeneration). Immunohistochemical staining was performed on paraffin sections (4μm) of the liver tissue. Two independent board-certified pathologists who were blinded to the study interpreted the histopathology (22).
3.7. Measurement of ALT, AST and Albumin in the Serum
Biocon standard kits and DAX-48 autoanalyzer were used to measure alanine aminotransferase (ALT), aspartate aminotransferase (AST), and albumin (ALB) activities in the serum, according to Wilkinson et al. and Bessay et al.’s method (23, 24).
3.8. Liver Glutathione Content
The liver samples were analyzed for their glutathione (GSH) content (25). The GSH contents were assessed by determining non-protein sulphydryl contents with the Ellman reagent (26). The liver samples (200 mg) were homogenized in 8 mL of 20 mM EDTA. Then, 5 mL of homogenized tissue was mixed with 4 mL distilled water and 1 ml of trichloroacetic acid (TCA) (50 % w/v). The mixture was shaken and centrifuged (15 minutes, 700 g, 4°C). Then, 2 mL of supernatant was mixed with 100 μL of Ellman reagent (DTNB, 0.01M in methanol), and the absorbance of color was measured at 412 nm with an Ultrospec 2000® UV spectrophotometer to obtain the value of GSH plotted calibration curve (27).
3.9. Determining Lipid Peroxidation in the Liver Tissue
The lipid peroxidation level was measured in the experimental groups. At first, 500 mg of liver tissue was gently minced in 4.5 mL of 0.25 M sucrose. The minced tissues were gently homogenized and then centrifuged at 380 g for 30 minutes. Then 0.1 mL of the supernatant was treated with a buffer containing 0.75 mL of thiobarbituric acid (0.8%, w/v), 0.75 mL of 20% acetic acid (pH = 3.5), and 0.1 mL of sodium dodecyl sulfate (8.1%, w/v). The solution was mixed up with 2 mL of distilled water, and heated in a boiling water bath for 60 minutes (26). Samples were centrifuged (3000 g for 5 minutes), and the absorbance of developed color was read at 532 nm, using an Ultrospec 2000 ®UV spectrophotometer to obtain the value of lipid peroxidation plotted calibration curve (28).
3.10. Statistical Analysis
All data were presented as Mean ± SD, and statistically significant differences were obtained between the control and experimental groups, using one-way analysis of variance (ANOVA) and Tukey’s as post hoc test, followed by Graph pad Prism 5. The Kruskal-Wallis test and Mann Whitney U test were employed for histopathological data comparison. The significance level was set at P < 0.05.
4. Results
4.1. Histological Analysis
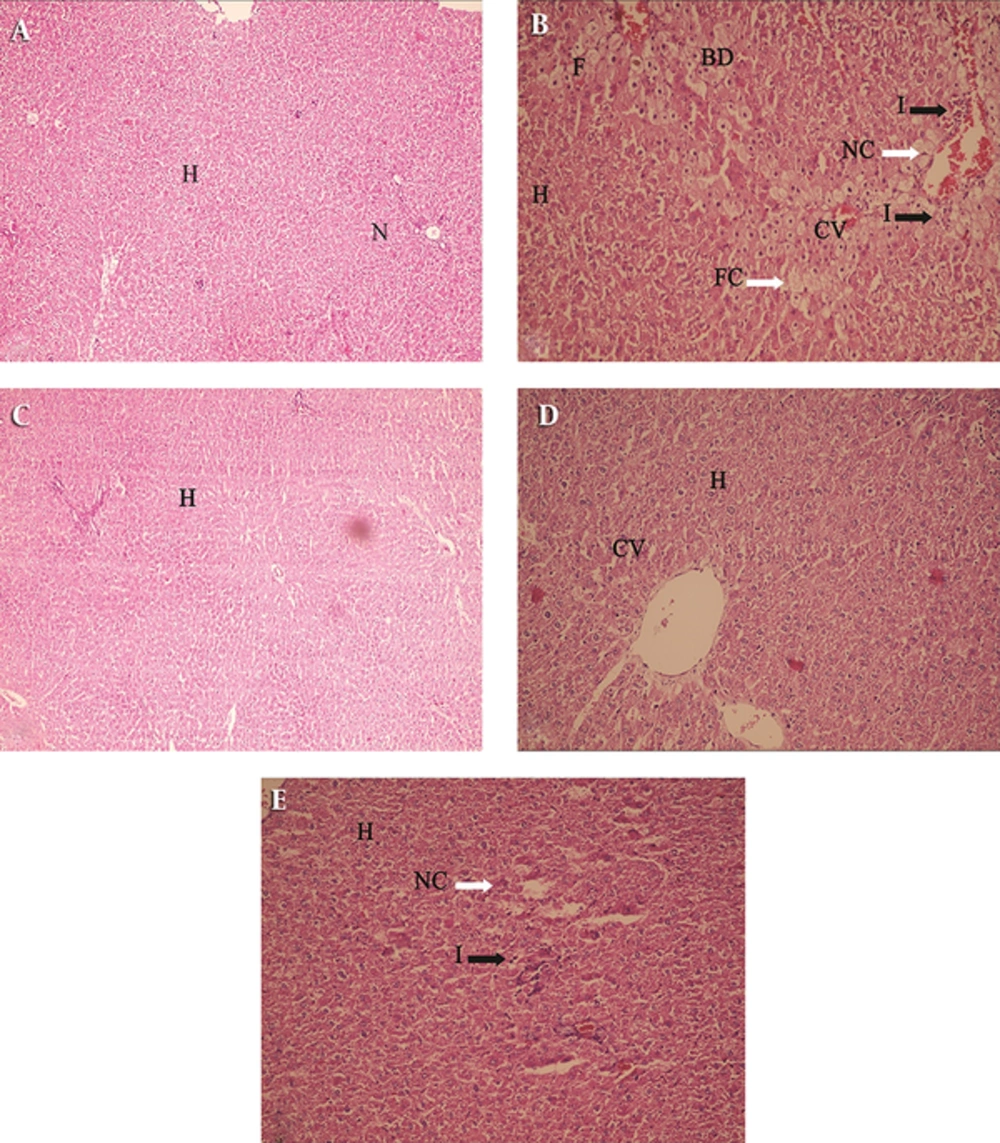
Histological studies of the liver sections from control rats revealed normal architecture, and were characterized by polyhedral shaped hepatocytes with small uniform nuclei. Hepatocytes were arranged in well-organized hepatic cords and separated by narrow blood sinusoids (Figure 1A). Hepatic sections, followed by CCl4 treatment, showed typical CCl4-induced liver injury (Figure 1B).The sections revealed extremely vacuolated hepatocytes (apoptotic necrotic cells and a lot of foamy cells) adjacent to the central vein (arrows and arrowhead, Figure 1B), indicating chronic CCl4-induced hepatotoxicity (with +++ grade in Table 1), moderate inflammation (with + grade in Table 1), and fatty changes (with + grade in Table 1).
A: (H & E × 100) Liver section of normal rats showing normal hepatocytes with prominent nucleus, cytoplasm and central vein; (B) (H & E × 250) Liver sections of CCl4 treated rats showing fatty chain, necrosis, infiltration of inflammatory cells; (C) (H & E × 250) Liver sections of the rats treated with MSC showing well brought out central vein hepatocytes with well-preserved cytoplasm (D) (H & E × 250); liver sections of the rats treated with PE showing well brought out normal hepatocytes with prominent nucleus (E) (H & E × 250); Liver sections of the rats treated with CCl4 + PE showing well brought out central vein hepatocytes with well-preserved cytoplasm, slight necrosis and Inflammation (H - Hepatocyte, CV - Central vein, N - Nucleus , F-foamy macrophage cells, FC - Fatty Chain, NC - Necrosis ,I - infiltration of inflammatory cells, BD- ballooning degeneration). A: Control (0.5 mL/kg olive oil i.p.) B: CCl4 (0.5 mL/kg i.p.) C: MSC (1 × 106 cell in 1 mL PBS infusion in liver) D: PE (0.5 mL/kg s.c.) E: CCl4 + PE (0.5 mL/kg i.p + 0.5 mL/kg s.c.)
| Groups | Ballooning Degeneration | Fatty Change | Hepatocyte Necrosis | Inflammation | Fibrosis |
|---|---|---|---|---|---|
| Control | 0 | 0 | 0 | 0 | - |
| CCl4 | ++ | + | +++ | + | Many foamy macrophages and old necrosis |
| MSC + CCl4 | 0 | 0 | 0 | 0 | - |
| PE | 0 | 0 | 0 | 0 | - |
| PE + CCl4 | 0 | 0 | + | + | - |
aRats were injected CCl4 with doses (1 mL/kg i.p. 1:1 in olive oil) twice a week for eight weeks. Four weeks after the first injection, MSC was infused to their livers locally, and then injection of CCl4 was continued for eight weeks. Values were considered as mean ± SD of seven rats per group; 0: Absent +: Mild ++: Moderate +++: Severe.
Comparing these groups, considering the hepatocyte necrosis, inflammation and fibrosis, it was found that ballooning degeneration was decreased in group III compared to group II, and the same result was obtained for group V. In group IV that received only PE, there was no significant toxicity, which shows that PE did not induce hepatotoxicity (Figure 1D). Fatty changes in both cell and PE administration were decreased compared to group II. Liver necrosis in both groups (groups III and V) was decreased, but better results were found in group III (0 grade in Table 1, and Figure 1C) compared to group II (+++ grade). Liver inflammation was better in group III (0 grade) compared to group V and group II (Table 1, Figure 1). In both groups, liver fibrosis was very low compared to group II.
4.2. Liver Enzymes and Albumin in Serum Analysis
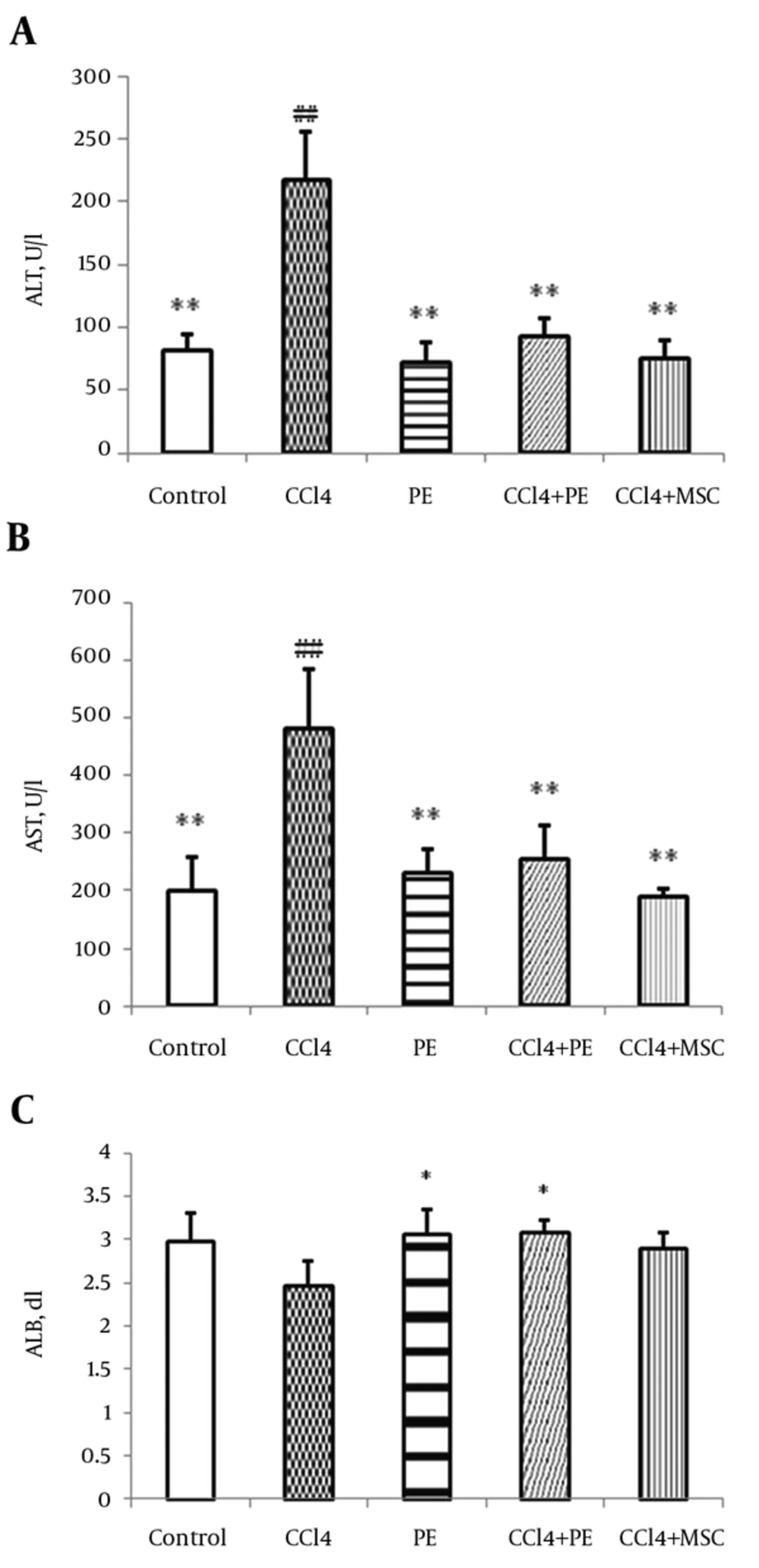
Liver enzymes showed significant changes. For instance, ALT enzyme revealed a statistically significant decrease in group III and in the group receiving platelet extract compared to group II (P < 0.01) (Figure 2A). A significant (P < 0.01) difference was obtained between the levels of ALT in groups I and II. AST enzyme also showed a statistically significant decrease compared to group II (Figure 2B) (P < 0.01). Moreover, a significant (P < 0.01) difference was found between the levels of AST in groups I and II. The results revealed a slight increase in serum albumin levels in group III, which was insignificant, but the increase was significant in group V compared to group II (Figure 2C). No significant differences were detected in the levels of albumin between groups I and II.
A: ALT, B: AST, C: Albumin (ALB). Rats were injected CCl4 with doses (1 mL/kg i.p. 1:1 in olive oil) twice a week for eight weeks. Four weeks after the first injection, MSC was infused to their livers locally and then injection of CCl4 was continued for eight weeks. Another group received PE subcutaneously five weeks after CCl4 was injected intraperitoneally. Values were considered as mean ± SD of six rats per group. *: Significantly different from CCl4-treated group (P < 0.05). **: Significantly different from CCl4-treated group (P < 0.01). ##: Significantly different from control group (P < 0.01).
4.3. Lipid Peroxidation Analysis
As displayed in Table 2, liver lipid peroxidation revealed a statistically significant increase in group II compared to the control group (P < 0.01). Moreover, a significant decrease was found in lipid peroxidation in group III and in the group receiving platelet extract compared to group II (P < 0.01) (Table 2); the lipid peroxidation levels was reduced in these two groups almost equally. There were no significant differences between the experimental and the control groups.
Abbreviations: GSH, reduced glutathione; TBARs, thiobarbituric acid reactive substances.
aValues are mean ± SD, (n = 7).
bP ≤ 0.05 Mean differences compared to CCl4-treated rats.
cP ≤ 0.01 Mean differences compared to CCl4-treated rats.
dP ≤ 0.05 Mean differences compared to CCl4+PE-treated rats.
4.4. Glutathione Analysis
GSH was also assessed as a liver antioxidant indicator. There was a significant increase in GSH levels in groups III and V compared to group II (P < 0.05). No significant differences were found between the experimental and the control groups. However, a significant increase was found in GSH levels in groups III and V compared to group II (P < 0.05). Between the two experimental groups, the cell receiving group showed a statistically significant increase compared to the platelet extract receiving group (P < 0.05), which represents the superiority of treatment with cells on the GSH content of liver and its antioxidant effects compared to treatment with platelet extract (Table 2).
4.5. Immunohistochemical Analysis
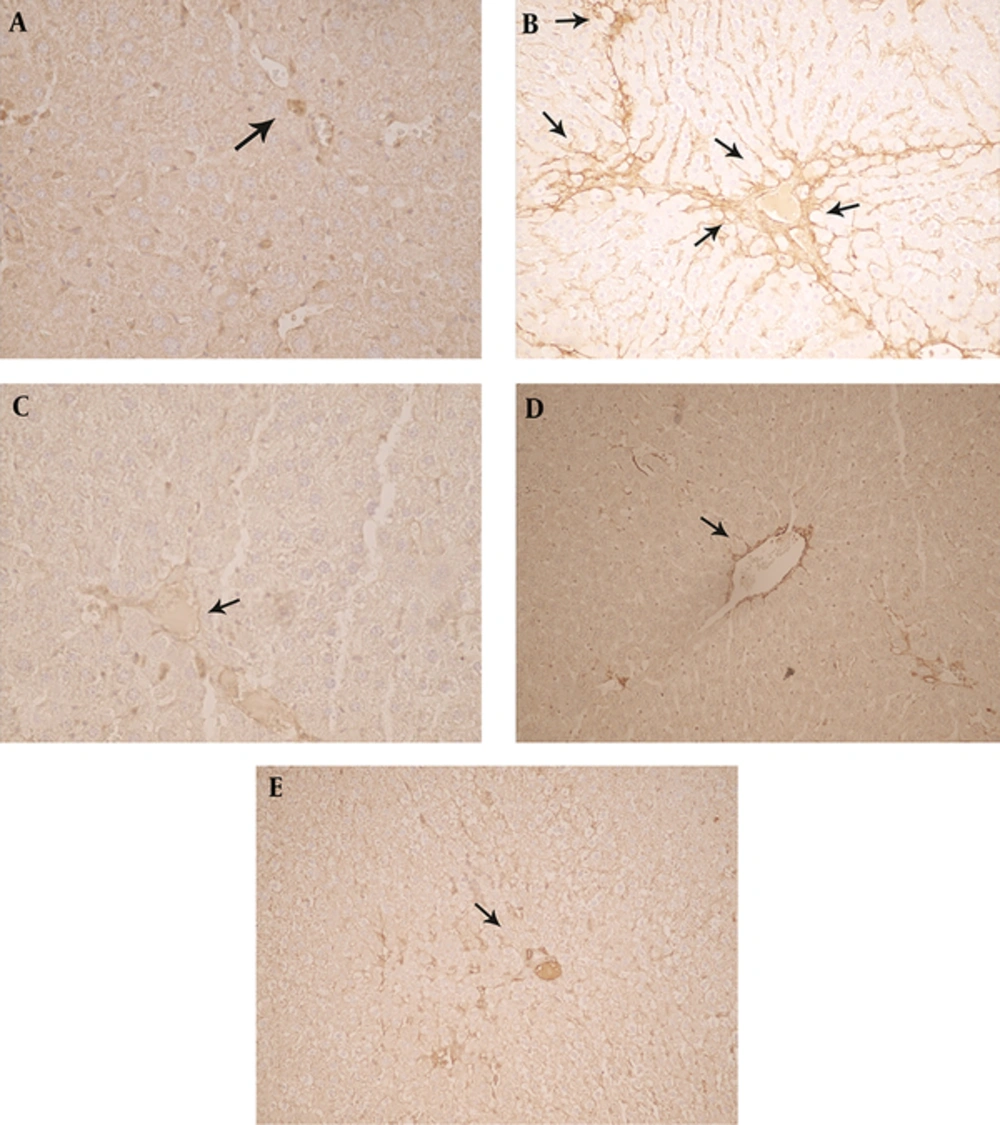
Immunohistochemical analysis of the liver tissue revealed that MSC and PE had modulatory effects on HSC (hepatic stellate cells) (Table 3 and Figure 3C, E) that are the major source of fibrillar collagens and other ECM, which characterizes liver fibrosis. Following a liver injury, HSCs undergo a phenotypic switch from quiescent and start to produce α-smooth muscle actin (α-SMA) positive, myofibroblast-like cells, a process termed activation. Activated HSC is central to liver fibrosis, and induction of HSC apoptosis is a potential antifibrotic treatment. Ki-67 was the other indicator examined in immunohistochemical tests, which represents the rate of cell division in hepatocytes. According to Table 3, it was determined that there was not any differences in the expression of this protein in the cell receiving group and platelet extract receiving group compared to group II.
aValues are mean ± SD, (n = 7).
bP ≤ 0.001 Mean differences compared to CCl4-treated rats.
cP ≤ 0.001 Mean differences compared to control rats.
(A) In the livers of control animals, α-SMA immunopositivity was restricted to smooth musculature (arrow), while other liver cells remained negative (B). In mice receiving CCl4 twice a week for eight weeks (0.5 mL/kgi.p. 1:1 in olive oil), many α-SMA containing myofibroblasts were strongly and diffusely stained in affected lobuli (arrows). (C) In the MSC + CCl4 group four weeks after the first injection, MSC was infused to their liver lobs locally and then injection of CCl4 was continued for eight weeks, clusters of moderately α-SMA immunopositive hepatocytes (arrows) (D). The rat livers received (0.5 mL/kg s.c.) PE (E). The rat livers receiving liver sections of the rats treated with CCl4 + PE (0.5 mL /kg i.p + 0.5 mL /kg s.c.) showed normal architecture of hepatocytes and mild infiltration of inflammatory cells. Original magnification 250 × immunohistochemical stain for α-SMA.
5. Discussion
Liver damage results from the excessive accumulation of extracellular matrix, which affects the liver function over time and leads to liver failure. The damaged liver increases the serum levels of transaminases (AST and ALT) and ALP because they are usually found in the cytoplasm and released into the circulation system during cellular damage (29). In the past, liver transplantation was thought to be the only treatment, but due to the shortage of proper donors, other medical treatments have been taken into consideration.
Some experimental rat models of liver injury indicated autologous transplantation of bone marrow-derived MSC in experimental rat models (30-32). Oyagi et al. (30), WU et al. (31), and Luk et al. (32) found that autologous transplantation of MSC can increase serum albumin levels, suppress hepatic transaminase activity, and reduce hepatic fibrosis in the experimental animals. Chemical damage or viral infection cause liver injury, and leads to liver fibrosis. Rats treated with CCl4 are usually used as an in vivo model for studies on liver damage. In our previous study, we investigated the therapeutic effects of human xenogenic MSC in CCl4 damaged rats. Local injection of xenograft MSC increased the level of albumin, and decreased the level of liver enzymes ALT, and AST (16). In this study, the comparative effects of MSCs and PE on CCl4-induced chronic liver injury was indicated. PE is widely applied in different clinical applications due to the major functions of activated platelets. Platelet administration 24 hours after hepatectomy via portal vein showed no significant changes in biochemical markers of liver function such as ALT and AST (33), whereas in this study, a significant decrease was detected in the function of liver enzymes after PE subcutaneous injection in group II. Moreover, serum albumin levels increased substantially after injection of PE (P < 0.05). Although treatment with MSCs and/or PE increased ALB level and decreased the function of liver enzymes, the comparison between cell therapy and therapeutic use of MSC was not significant in this study (Figure 2A, 2B, 2C).
CCl4 is converted to free radicals by Cytochrome P-450, which applies its effects on liver through lipid peroxidation (34). Cell damage occurs when reactive oxygen species increase in the liver. These oxidants can destroy cells by beginning chemical chain reactions such as lipid peroxidation or by oxidizing DNA (35).The use of antioxidants reduces the amount of these free radicals.
Glutathione (l-γ-glutamyl-l-cysteinylglycine) provides the reduction capacity and plays a very important role in the detoxification of free radicals (36). GSH was also assessed as an indicator of liver antioxidant function. Table 2 demonstrates the effect of therapeutic use of the bone marrow-derived MSCs and PE on GSH and thiobarbituric acid reactive substances (TBARs) levels of the liver damaged by CCl4 in rats. A significant increase was observed in GSH levels in groups V and III compared to group II (P < 0.05). Reactive oxygen production, followed by direct effects of toxic and hydrophobic bile acids on the mitochondria of hepatocytes, leads to necrosis and apoptosis of hepatocytes (37). The glutathione level as an intrinsic antioxidant increased significantly in group III compared to groups t I, II and V (Table 2). Although treatment with MSCs and/or PE increased the GSH level, the comparison between cell therapy and therapeutic use of PE was significant (P < 0.05) in increasing the level of GSH; MSCs had a better effect on the GSH content of the liver compared with PE. To the best of our knowledge, this was the first study to demonstrate the comparative effects of MSC and PE therapy by GSH index.
Hepatic fibrosis is the common pathological change of chronic liver diseases that can progress to liver cirrhosis .The activation of hepatic stellate cells (HSCs) is noted as the central event of hepatic fibrosis; thus, the common therapy for hepatic fibrosis was targeted to these cells and attained substantial efficacy in animal experiments. In this study, the immunohistochemical analysis revealed that both MSC and PE could decrease SMA in the liver due to reduction of hepatic fibrosis (Table 3, Figure 3). The fact that MSC has anti-fibrosis effects on the damaged liver is obviously specified in animal models of liver fibrosis (38, 39). MSC has an inhibitory effect on the liver fibrogenesis, which acts by inhibiting HSC and resetting fibrogenic process (40).
Table 1 show the effect of the marrow derived MSCs and PE on histopathological liver damages induced by CCl4 in rats. The histopathology comparison in terms of the characteristics of inflammation, hepatocyte necrosis, fatty change and fibrosis revealed a significant decrease between groups V and II where the positive effects of subcutaneous injection of platelet extracts were confirmed (Table 1; Figure 1E). Platelets reinforce hepatocyte regeneration and have an anti-fibrotic effects in vitro (41) and in vivo (42). Platelets and their extracts have therapeutic effects on their growth enhancing and wound healing (43). The results of this study revealed that PE had a good effect on liver fibrosis induced by CCl4, and reduced α- SMA in the liver injury (Table 2, Figure 2).
The other immunohistochemistry marker, Ki-67, was evaluated as an indicator of liver cell division. Matsuo, R. et al. found that Ki-67 marker was observed 24 hours after the liver damage (33). Murata and colleagues determined that a 2-3 fold increase in the number of platelets which was induced by thrombopoietin led to an increase in the liver weight/body weight ratio, Ki-67 marker in hepatocytes, and mitotic index after removal of 70% of the liver (44). Comparing the two experimental groups (III and V), no significant changes were observed (Table 3). Subcutaneous injection of PE did not increase Ki-67 marker in the rat liver toxicity induced by CCl4 in this study. It has been suggested that cell transplantation and platelet extract improved the function of the liver cells only by reducing the HSC levels (α-SMA) and decreasing apoptosis and fibrosis rates of the liver cells, which did not enhance liver cell division.
5.1. Conclusion
Our results provide evidence for the protective effects of both MSCs, and PE on CCl4-induced liver toxicity in rats. Both cell and platelet therapy methods not only have a direct effect on eliminating reactive oxygen species, they may also affect endogenous antioxidants such as GSH. Moreover, local injection of human xenogenic MSCs, and subcutaneously allogenic injection of PE could increase the serum albumin level and decrease the function of liver enzymes. It has been suggested that these methods could be a therapeutic approach for liver damage, particularly for the damages associated with oxidative stress.