1. Background
Interest in biodegradable packaging from aquaculture, agriculture, and cattle farming materials, such as proteins, lipids, and polysaccharides, is currently increasing because of the health concerns of consumers and the environmental problems posed by plastic packaging wastes (1, 2). Films made from plant and animal protein sources include corn zein, wheat gluten, soy and peanut protein, cotton seed, albumin, gelatin, collagen, casein, and whey proteins (3). Studies have addressed the blending of two or three kinds of materials to improve the physico-chemical properties of films, such as pullulan (linear homopolysaccharide of glucose) with chitosan and carboxymethyl chitosan (4) or with sodium caseinate (5) chitosan with soybean trypsin inhibitor (6). However, the use of proteins and polysaccharides is limited because of their poor mechanical properties and high sensitivity to moisture. Earlier studies showed that the blending of pullulan and SPC at a ratio of 50: 50 could improve mechanical properties and water vapor permeability.
Antimicrobial packaging technique as an active packaging technique is one of the new approaches to overcoming decay and disease outbreaks caused by microbial growth problems. Thin layers of biopolymers can be incorporated into antimicrobial agents and coated on the surface of foods and drugs against microbial contamination to increase shelf life and maintain quality (7). Antimicrobials, antioxidants, and other additives from natural sources are acceptable for consumers. Different parts of plants contain essential oils (EOs) with various chemical compositions (mainly phenolic components), which can differ depending on the harvesting season, geographical source, and function of a part from the same plant. These natural extracts are volatile oily liquids with antibacterial, anti-fungal, anti-viral, insecticidal, and antioxidant properties (2, 8).
Zataria multiflora (ZM) or Avishan Shirazi (Iranian local name) is a plant that grows only in Iran, Pakistan, and Afghanistan. This plant is widely used as flavoring agents in food processing and many pharmacological preparations, such as antiseptic, anesthetic, and antispasmodic. The main antimicrobial constituents of this plant are phenolic compounds such as carvacrol and thymol (9).
The Artemisia genus includes 15 perennial aromatic herbs and shrubs that grow wild in dry or semi-dry habitats. These plants produce non-phenolic and phenolic EOs and are used as spices and antiseptics in folk remedies. Previous research showed that the main components of the EO of Artemisia biennis (AB), which is grown in Iran, are alpha-pinene (10.2%), 1, 8-cineole (10.1%), artemisia ketone (11.4%), and camphor (24.6%) (10).
2. Objectives
The study aimed to develop biodegradable films containing ZM and AB EOs. Some selected properties of these films were analyzed, including thickness, tensile strength, elongation at break, and water vapor permeability. The effectiveness of composite films containing EOs was determined against three pathogens, namely, Escherichia coli, Staphylococcus aureus, and Lactobacillus plantarum. The minimum inhibitory concentration (MIC) of EOs for each bacterium was also determined.
3. Methods
3.1. Materials
Pullulan, soy protein concentrate (SPC; defatted low-heat soybean meal) with 85% protein content on a dry basis, and two types of commercial EOs extracted from the leaves of ZM and Artemisia sieberi were supplied by Hayashibara Company (Tokyo, Japan), Tianjing Corporation (China), and Barij Essence Company (Iran), respectively. Glycerol plasticizer (a food grade reactant), sodium hydroxide, magnesium nitrate, and calcium chloride were purchased from Merck KGaA (Darmstadt, Germany). The medium for microbial culture, which contains MRS broth, tryptic soy broth, and nutrient agar, was supplied by Merck Company (Germany).
S. aureus (PTCC 1189), L. plantarum (PTCC 1058), and E. coli (PTCC 1399) cultures were purchased from Tehran University collections (Iran) on tryptone soy agar slants.
3.2. Preparation of Composite Films With EO
The pullulan and SPC solution were prepared by dissolving 5% of pullulan or SPC (w/v) in distilled water with glycerol (3.5% wt/vol). The pH of the SPC solution was adjusted to 10.0 with 0.1N NaOH and heated up to 90°C on a hotplate stirrer for 30 minutes (11, 12). In this study, the pullulan and SPC solutions were mixed at a 50: 50 ratio.
The temperature of pullulan and SPC solutions was maintained below 20°C to avoid the destruction of sensitive EOs. Then, EOs from ZM or AB were incorporated into the film solution at various concentrations of 0% (50: 50 ratio of SPC with pullulan film as the control), 5%, 10%, 15%, and 20% (v/v) of edible film. The casting/solvent evaporation method was used for film formation. Solutions of 10 mL were casted onto sterile plastic petri dishes and allowed to dry in the oven at 30°C for 48 hours. Films were peeled and stored in a vacuum desiccator for further use.
3.3. Film Thickness Measurement
Thickness was measured at 10 random places at least in the film sheets with a micrometer (Mitutoyo, USA) to the nearest 0.001 mm. The thickness of an average film was used for tensile properties and water vapor permeability (WVP) tests.
3.4. Water Vapor Permeability
WVP of films was measured according to the ASTM E96 method with a 75% relative humidity (RH) gradient at 25°C with slight modifications (13). Glass cups containing anhydrous calcium chloride were sealed with films over the rim of the cup by applying molten paraffin. The cups were placed in a desiccator to maintain saturated normal saline. The cups were weighed to determine the amount of water that permeated through the films every 1 hours for 24 hours. The gradient was obtained from the slope of a linear regression of the weight increase versus time (Microsoft® Office Excel 2007). WVP was calculated according to Equation 1:

Where TH is the average film thickness (mm) and /Δp is the gradient of the partial vapor pressure across the film in Pa (1753.55 Pa at 25°C). WVP (gm-1 s-1 Pa-1) was made in triplicate samples for each type of film (13).
3.5. Water SOLUBILITY
The percentage of film dry matter soluble after 6 hours immersion in distilled water was obtained in triplicate and referred to as water solubility. Film portions were cut (2 cm × 2 cm) and dried in the oven at 105 ± 2°C for 24 hours and weighed to obtain the initial dry weight. Dried films were dropped into an Erlenmeyer flask (250 mL) containing 50 mL distilled water, and the system agitating periodically for 6 hours at 25°C. Then, the film samples were passed through a filter paper (Whatman 1), and the insolubilized fraction was dried at 105 ± 2°C until constant weight (final dry weight). Water solubility was calculated using Equation 2 (13).

3.6. Mechanical Properties
The average film thickness was inputted into the texture analyzer (Gotech, Taiwan) to determine tensile strength (Mpa) and percentage elongation at break (%) according to the ASTM method D882 (14) with slight modifications. The films were cut into strips (10 mm × 60 mm) and conditioned in a desiccator containing a saturated solvent of magnesium nitrate at 25°C for 24 hours prior to testing. The film samples were fixed between the grips with an initial separation of 50 mm at 20°C and 65% RH in a controlled room, and the cross-head speed used was set to 50 mm/min. The values of tensile strength and percentage elongation were shown on the monitor of the texture analyzer at the end of the test. All of the results were the means of at least three measurements (13, 14).
3.7. Antibacterial Activity Test
The perpetrated films were cut into disks of 6 mm diameter by a circular knife for the antibacterial activity test using the agar diffusion method. L. plantarum was cultured in MRS broth, while E. coli and S. aureus were cultured in tryptic soy broth. Both cultures were grown at 37°C for 24 hours. The surface of the nutrient agar plate was spread with the inoculums of L. plantarum, E. coli, and S. aureus in the range of 105 - 106 CFU/mL, and the cut films were placed on them. The plates were incubated at 37°C after 24 hours, after which the diameter of the inhibitory zone surrounding the film disks was measured. If a surrounding clear zone was not present, it would be assumed that the essential oil concentration was not inhibitory and the area would be assigned with a zero value. The MIC was determined by the following definition: the lowest EO concentration resulted in the lack of visible micro-organism growth. In addition to the initial concentrations (20%, 25%, 30%, and 35%) for the determination of MIC, films with 5%, 10%, and 15% were also prepared.
3.8. Statistical Analysis
The triplicate data (WVP, water solubility, mechanical properties, and microbial data) and the 10 replicates of thickness were processed by ANOVA to test the significance of the added EOs using the SAS program (Version 9.1; Statistical Analysis System Inst. Inc., Cary, NC, USA).
Analyses on the completely randomized design of Duncan’s one-way ANOVA were conducted to compare the differences among the means at the 0.05 level. All of the averages with standard deviation were presented.
4. Results
4.1. Film Thickness
As shown in Table 1, both EOs increased the thickness of films compared with the control film (pullulan-SPC blended film without incorporated EOs), but the means of the various concentrations were not significantly different (P < 0.05). The ZM EO increased the thickness of films more than the AB EO, but this increase was not significant (P < 0.05).
| EOs | Thickness, mm | WVP (× 10-11 gm-1s-1Pa-1) | Solubility in Water (%) | Tensile Strength, MP | Elongation at Break (%) |
|---|---|---|---|---|---|
| Control film | 0.121 ± 0.0157A | 5.01 ± 0.0168A | 81.13 ± 3.43A | 4.11 ± 0.46A | 209.98 ± 6.24C |
| Z20 | 0.136 ± 0.0097A | 4.26 ± 0.143A,B | 68.91 ± 0.45B,C | 1.85 ± 0.11CD | 223.91 ± 8.83B,C |
| Z25 | 0.139 ± 0.0025A | 4.11 ± 0.203B,C | 65.21 ± 5.03C,D | 1.66 ± 0.45D,E | 243.11 ± 7.65B,C |
| Z30 | 0.141 ± 0.002A | 3.2 ± 0.144D,E | 65.03 ± 3.52C,D | 1.24 ± 0.19E | 248.11 ± 10.4A,B |
| Z35 | 0.146 0± .00025A | 2.63 ± 0.33E | 62.01 ± 2.56B | 1.22 ± 0.17E | 269.67 ± 6.03A |
| A20 | 0.145 ± 0.013A | 4.42 ± 0.396A,B | 74.16 ± 1.67B | 2.8 ± 0.64B | 219.76 ± 9.24B,C |
| A25 | 0.145 ± 0.003A | 4.38 ± 0.364A,B | 73.15 ± 0.06B | 2.45 ± 0.09B,C | 229.31 ± 0.24B,C |
| A30 | 0.147 ± 0.004A | 3.63 ± 0.403C,D | 71.08 ± 3.72B | 2.26 ± 0.06B,C | 237.19 ± 12.47A,B |
| A35 | 0.151 ± 0.00015A | 2.86 ± 0.662E | 69.93 ± 0.89B,C | 1.92 ± 0.09C,D | 244.26 ± 8.7A,B |
aData are presented as the mean values of three replicates (for thickness, the mean values of 10 replicates) standard deviation.
bDifferent superscript capital letters in the same column indicate significant differences (P < 0.05).
4.2. Water Vapor Permeability
WVP of all film types was measured and is shown in Table 1. The addition of EOs reduced the WVP of the films significantly (P < 0.05). The ZM and AB EOs had the same effect on decreasing WVP in most concentrations.
4.3. Water Solubility
Solubility of the pullulan-SPC films incorporated with ZM and AB EOs at various concentrations is presented in Table 1. The control film showed the highest film solubility (81.13%) as both SPC and pullulan are water sensitive, whereas the films incorporated with ZM and AB EOs exhibited low water solubility (P < 0.05), which is attributed to the integration of the film network.
4.4. Mechanical Properties
The tensile strength (TS) and elongation at break (EAB) of pullulan-SPC films incorporated with AB and ZM EOs at various concentrations are shown in Table 1. The control film had the highest TS (4.11 Mpa) and the lowest EAB (209.98%), thus making this film the toughest one. The results showed that in the case of the films with EOs, TS decreased and EAB increased when the concentration of EOs increased. However, AB and ZM EOs seemed to produce weak films, which were more elastic and flexible than the control film. The TS and EAB of the resultant films were more affected by ZM than by AB. As shown in Table 1, films with 35% ZM EO had the highest EAB (269.67%) and the lowest TS (1.22 Mpa), For films with added AB EO, the significant decrease in TS was 2.8 - 1.92 Mpa for 20% - 35% EO concentration (P < 0.05).
4.5. Antibacterial Activity Test
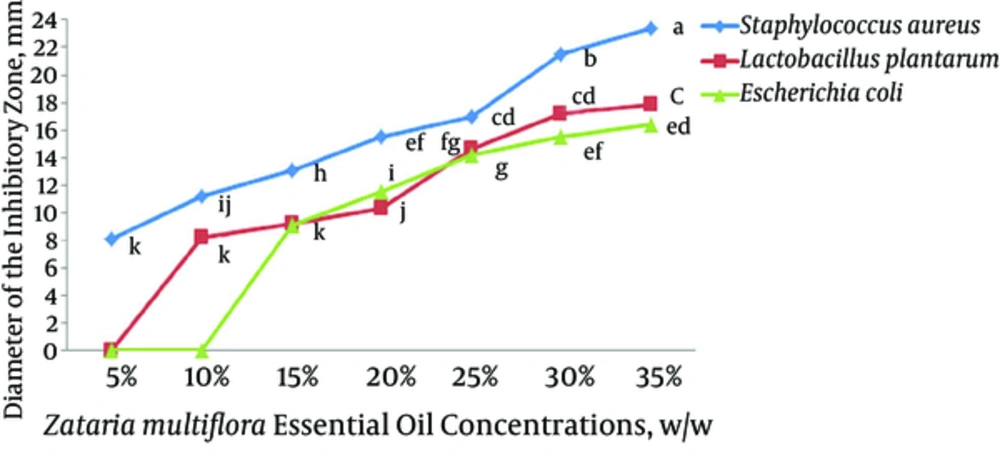
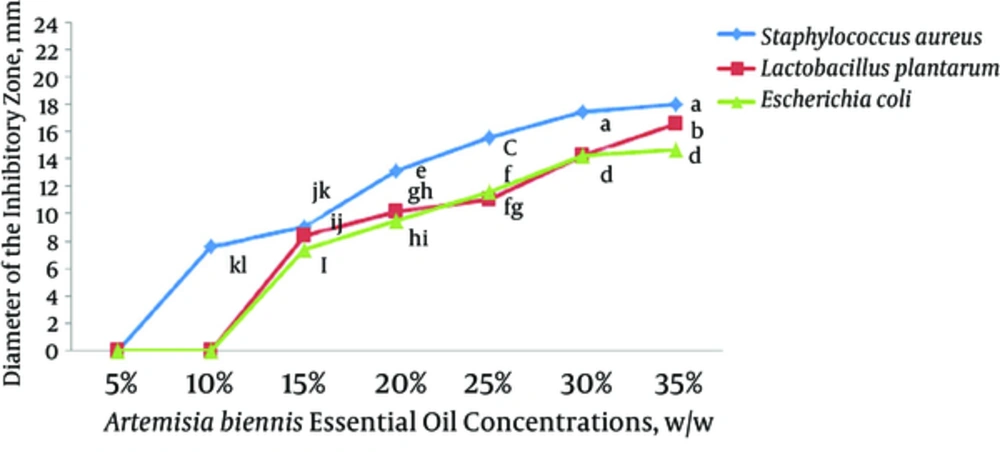
The antibacterial activity of ZM and AB EO films against L. plantarum, E.coli, and S. aureus is illustrated in , respectively. Both ZM and AB EOs exhibited a significant antibacterial activity against L. plantarum, E.coli, and S. aureus (P < 0.05) but in various degrees. Among the tested bacteria, E. coli and S. aureus had the highest and least resistance to the antimicrobial effect of EOs, respectively. An overall view of the results showed that the antibacterial activity of EOs increased with the increase in EO concentration. The intensity of antimicrobial activity of ZM EO was significantly higher than that of AB EO at all concentrations (P < 0.05). The MIC for L. plantarum, E. coli, and S. aureus was determined to be 10%, 15%, and 5% for ZM EO and 15%, 15%, and 10% for AB EO, respectively.
5. Discussion
Pullulan-SPC films incorporated with EOs were homogeneous, flexible, easily removed from the plastic petri dishes without naked eye, and easily crack. The ZM EO increased the thickness more than the AB EO. This outcome could be related to the differences in molecular size and structure of these two EOs. The same results were reported for fish skin gelatin film incorporated with citrus (bergamot, kaffir lime, lemon, and lime) EOs. The increase in thickness was regardless of the type of EO incorporated (15). Hydrophilicity is one of the main factors affecting WVP in biodegradable films, especially the protein and polysaccharide polymeric film network (5). The addition of EOs reduced the WVP of the films. This effect is related to the great hydrophobicity of films containing different EOs. The capability to avoid moisture transfer between packaging material and the surrounding atmosphere is one of the important functions of biodegradable films. AB and ZM EOs have the same effects of decreasing WVP in most concentrations, and thus they improve and increase the resistance of films to moisture penetration. The same result was observed when hake protein films were incorporated with citronella, coriander, tarragon, and thyme oils. As shown in this study, the WVP values of hake protein film decreased with the increase in EO concentration. The maximum permeability reduction in the film prepared with thyme oil might have been caused by the greasier nature of the molecules (i.e., the greater hydrophobicity) of thyme oil compared with those of other oils, and this quality prevented the penetration of water molecules through the films. Therefore, the stable condition of the packaged products is preserved (16). Solubility in water can exhibit water resistance and integrity of biodegradable films when applied for the protection of foods and edible packaging (17). When the concentration of EOs increased, the solubility of the resultant films decreased but no significant difference was found in AB EOs at 20%, 25%, and 30% concentrations (P < 0.05). As ZM EOs have a hydrophobic nature and are greasier than AB Eos, they significantly decreased film solubility further (P < 0.05). The integration of film network by greasy EOs decreased the amount of water molecules present between polymer chains joined through hydrogen bonding. The same result was observed in the gelatin film from the skin of a unicorn leatherjacket incorporated with bergamot and lemongrass oil. The addition of bergamot and lemongrass oil could decrease the solubility of gelatin films. According to the non-polar components of oil and the hydrophobic domain of gelatin, hydrophobicity of the resultant films increased and thus the solubility of films decreased (17). However, films with EOs are less soluble in water than the control film. This property of the resultant films makes them a good alternative to edible films for packaging products. In ambient temperature, most of the EOs are liquid and their presence in the film structure in the form of oil droplets can make them easily deformed, can increase the film flexibility, and can have a plasticizing effect (17). This modification can facilitate the best mechanical performance for packaging. The results suggested that the incorporation of EOs caused the development of a heterogeneous and irregular film structure that could reduce cross-linking between polymers. TS and EAB of chitosan films incorporated with thyme, clove, and cinnamon EOs were determined. The incorporation of thyme and clove caused TS to decrease and EAB to increase significantly (P < 0.05) against cinnamon. The reduction in TS is attributed to the breaking up of the film network, and the enhancement of EAB might have been due to the increase in moisture content of these films. However, a strong interaction was observed between cinnamon and the polymer that decreased the free volume and molecular mobility of the polymer, thus causing a decrease in EAB. The decreased moisture content of chitosan films incorporated with cinnamon EO during the film production might have been due to the increase in TS (18). Gram-positive bacteria are more sensitive to EOs than gram-negative ones. This finding could be related to the structure of the bacterial membrane. In gram-negative bacteria, outside the cytoplasmic membrane is a peptidoglycan layer that restricts the diffusion of hydrophobic compounds. However, gram-positive bacteria do not have this layer, and thus external agents can permeate easily (19). According to previous studies, the main ZM EO phenolic compounds (i.e., carvacrol and thymol) have high levels of antimicrobial activity (9, 20). The lethal mechanisms of phenolic compounds are expressed by destabilizing the cytoplasmic membrane attacks to the phospholipid cell membrane. This destabilization increases the permeability and leakage of cytoplasm interaction with enzymes located on the cell wall and in the exchanged proton, thereby reducing the pH gradient across the membrane, collapsing the proton motive force, and discharging the ATP pool (19, 21). The antimicrobial activity of gelatin chitosan films incorporated with clove, fennel, cypress, lavender, thyme, herb-of-the-cross, pine, and rosemary EOs were examined against 18 genera of bacteria. Clove EOs were the most effective ones, followed by rosemary and lavender. However, clove and thyme EOs were the most effective in fish extract. Thyme EO was inhibited by S. aureus more than by E. coli consistent with the result of this study (2). Seven species of Artemisia, (i.e., Artemisia absinthiumL., Artemisia biennis Willd., Artemisia cana Pursh, Artemisia dracunculusL., Artemisia frigida Willd., Artemisia longifolia Nuttall, and Artemisia ludoviciana Nutt) were studied to determine the inhibitory effects of Artemisia on the growth of E. coli, S. aureus, Staphylococcus epidermidis, Candida albicans, Cryptococcus neoformans, Trichophyton rubrum, Microsporum canis, Microsporum gypseum, and Aspergillus niger. E. coli and C. albicans had minimal growth inhibition zones and minimal susceptibility to the EOs. In all tested species, the growth inhibition of S. aureus was higher than that of E. coli. The volatile components analyzed from these EOs showed high amounts of oxygenated monoterpenes with 1, 8-cineole, camphor, camphene, alpha-pinene (monoterpenene hydrocarbon fractions) artemisia ketone, and artemisia alcohol (10, 22).
5.1. Conclusion
In this study, pullulan and SPC solutions were incorporated with ZM or AB EOs at various concentrations. The obtained films were flexible and free of pores. Compared with the control film, the films added with EOs had significantly amplified thickness and EAB but decreased TS, WVP, and solubility in water (P < 0.05). The EOs had significantly more antimicrobial activity against S. aureus than against E. coli (P < 0.05). This new antibacterial film can be synthesized for various applications in food and drug packaging.