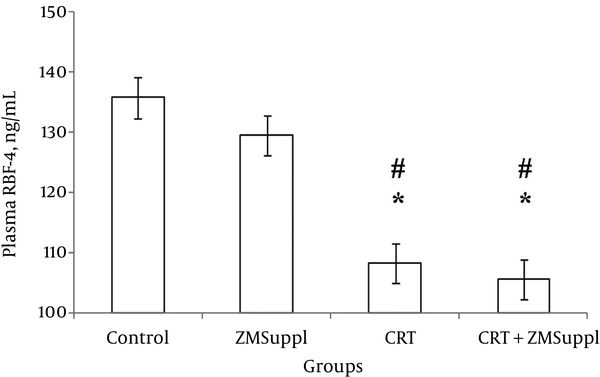1. Background
Adipokines, substances that are directly produced by fat cells, affect inflammatory responses cause insulin resistance and increase the risk of cardiovascular diseases (1).
A large number of studies confirmed that the relationship between circulation levels of retinol binding protein-4 (RBP-4), an adipokine, is derived from adipocyte, various aspects of obesity (2), insulin resistance and sensitivity (3), type 2 diabetes (4) and metabolic syndrome (5). It seems appropriate to reduce insulin sensitivity to resistance and risk factors of cardiovascular diseases in different patients (6).
Tumor necrosis factor-α (TNF-α), another adipokine related to obesity and insulin resistance (7, 8), is a multi-functional cytokine that can regulate many cellular and biological processes, such as immune function, cell differentiation, proliferation, apoptosis and cell metabolism. Many studies reported increased serum TNF-α in obesity, while weight loss is associated with its reduction (9).
On the other hand, menopause is a phenomenon associated with aging in females (10). The development of abdominal obesity and as a result increased incidence of metabolic syndrome and type 2 diabetes in females after menopause are mainly associated with loss of the protective role of estrogen and a relative increase in androgen circulation (11). Also, in menopause, decreased circulation of sex hormones causes pro-inflammatory activities (12). In addition, menopause increases obesity that plays a role in systemic inflammation (12).
Nowadays, the importance of exercise in reducing inflammation and inflammatory factors is considered more (13). Exercise increases the blood flow, capillary density, muscle mass, glycogen storage capacity due to increased glycogen synthase, increase in muscle glucose transport protein (GLUT4), improving and increasing glucose uptake rate by muscles, improving insulin sensitivity and efficiency, reducing insulin resistance, increasing gene expression or activities of different proteins involved in insulin signaling cascade, and reduces weight and RBP-4 concentration in untrained people and those with impaired glucose tolerance and diabetes (14-16).
But recently, resistance training (RT) or weight training is a common means to improve health and increase muscle mass (16). RT is a treatment program by American heart association (AHA) (17) and American college of sports medicine (ACSM) (18). A study showed that muscle hypertrophy due to RT was associated with lower levels of inflammatory markers and cytokines (19). In addition, RT with high intensity may compensate for reduction in bone mass and muscle strength regarding age in postmenopausal females (20). Traditional RT causes increase in strength, fat free mass, bone density, and also improvement in blood lipid, but has no significant effect on cardio-respiratory endurance. But, RT in circuit style (circuit resistance training/CRT) causes improvement in cardio-respiratory endurance and can be a kind of exercise to improve both strength and cardio-respiratory endurance (21).
On the other hand, the proper combination of diet and exercise can have a positive effect on health-related quality of life than any of them alone (22). New researchers tended to use herbal medicines with regard to complications caused by chemical medicines. Zataria sp. is a plant belonging to Labiatae family with the scientific name Zataria multiflora (ZM) that grows only in Iran, Pakistan and Afghanistan (23). ZM, due to its excellent properties, is widely used as tea, spices, and herbal medicine is widely used to treat asthma and is used as an antiseptic of stomach and urinary tract as well as diuretic and anti-inflammatory in traditional medicine (24). ZM contains phenolic compounds such as carvacrol, thymol, and gamma-terpinene with anti-inflammatory effects (23, 25). Research shows that compounds containing ZM can inhibit the reactions of inflammatory mediators and act as an antioxidant, antispasmodic, and anti-pain (25). In a study, ZM was effective in the treatment of primary dysmenorrhea (26). Recently, in animal models, thymol and carvacrol acted as bioactive compounds reducing pre-inflammatory cytokine levels of interleukin (IL)-1β, IL-6, and TNF-α and increasing anti-inflammatory cytokines (27, 28). Also, a recent study showed that ZM can increase adipokine level, which may be due to increased gamma peroxisome proliferator-activated receptor (PPAR) protein (23). These effects may contribute to control the symptoms of menopause via anti-inflammatory effects and probably the balance of lipid tissue adipokine still is not investigated.
2. Objectives
Based on the symptoms of menopause, it is necessary to find new ways to decline or even control it; according to the literature about physiological and mechanical effect of CRT (in comparison with traditional RT) for menopausal females and anti-inflammatory effects of ZM, it seems that its parallel effect is helpful for females who developed menopause. Therefore, the current study aimed to investigate the effect of eight weeks of CRT with ZM supplementation (ZMSuppl) on RBP-4 and TNF-α in postmenopausal females.
3. Methods
The study was semi-experimental with pretest and post-test design. The study was approved by the research ethics committee of the Iranian sport sciences research institute, and conducted in accordance with the Declaration of Iranian ministry of health.
Subjects Eighty eight postmenopausal females volunteered through public call in public centers and offices in Tehran. Postmenopausal status of females was specified based on age range from literatures and also an interview by a gynecologist. Before participating in the study, all procedures were explained to the volunteers and after complete awareness of the study terms and the completion of a medical questionnaire, a written informed consent was obtained from them. Inclusion criteria were passing at least six months from menopause development, lack of addiction to drugs and alcohol, lack of regular exercise history for at least six months, no history of renal, hepatitis, cardiovascular disease, diabetes and/or any physical injury or problem as a physical activity obstacle. After examination by a gynecologist, the subjects enrolled into the study. Among them, 48 subjects with the age range of 48 - 65 years were randomly selected after approval by a cardiologist. Then, based on weight, height, and body mass index (BMI), subjects were matched and divided into four groups (n = 12): control, ZMSuppl, CRT, and CRT combined with ZMSuppl (CRT + ZMSuppl) (Table 1).
| Groups | Control | ZMSuppl | CRT | CRT + ZMSuppl |
|---|---|---|---|---|
| Age, y | 56.5 ± 4.2 | 54.4 ± 3.9 | 58.03 ± 4.7 | 53.8 ± 6.0 |
| Height, cm | 156.7 ± 3.0 | 160.9 ± 4.0 | 158.7 ± 3.8 | 159.2 ± 4.6 |
| Weight, kg | 68.7 ± 13.3 | 66.4 ± 10.9 | 67.8 ± 13.01 | 70.1 ± 10.8 |
| BMI, Kg/m2 | 28.27 ± 4.2 | 25.93 ± 2.5 | 27.22 ± 1.8 | 27.81 ± 2.0 |
Demographic Characteristic of Participantsa
3.1. Preparation of ZM Essence
ZM leaves were collected in March around the gardens of Eghlid city, Iran. Then, they were dried in the shade for 10 days. ZM leaves were dried in an oven for 48 hours at 32°C and then powder in a Chinese pounder. A 50 g of the sample powder was boiled for 3 hours using water distillation method in Clevenger and at boiling point. The essential oil was filtered, then dried on anhydrous sodium sulfate, and finally transferred into a capped glass container and stored at 4°C. The yield was calculated as dry essential volume divided by the initial dry powder weight multiplied by 100. The calculated yield was 3% (29).
3.2. Combination Assessment of ZM Essence by Gas Chromatography-Mass Spectrometry (GC-MS)
To isolate and identify components of ZM essence, GC-MS was used. GC-MS analysis was conducted by Agilent 5975 mass spectrometer detector (MSD) coupled with gas chromatography model of Agilent USA GC 7890A MS 5975C. The used column was of welded silica HP-5 (5% phenyl, 1.95% polydimethyl siloxane) with the profile of 30 × 0.25 mm2 id and film thickness of 0.25 μm. Helium was used as the carrying gas, and flow rate of gradient was 1 mL/minute. The thermal program was as follows: First, the column temperature was set at 50°C for five minutes, then it was increased 3°C per minute to 240°C, then it was increased 5°C per minute to 300°C, and finally set for three minutes at this temperature. The stored essential oil samples were diluted by n-hexane at a ratio of 1:10 and 1 µL was injected in the gas chromatography. The temperature at the injector and detector was fixed at 290°C. Compounds in ZM essence were identified using fragmentation pattern in the database of wiley7n.l and NIST08 and also using retention time in chromatography column (29). For each combination, the ratio of the level below peak was the total levels below peak of all compounds and the results are summarized in the Table 2.
| Compound | Inhibition Time, min | Under Peak Level, % |
|---|---|---|
| Thymol | 35.90 | 26.8 |
| Carvacrol | 36.50 | 22.9 |
| P-cymene | 21.73 | 7.7 |
| γ-Terpinene | 23.55 | 6.8 |
| α-Pinene | 16.53 | 3.2 |
| β-Caryophyllene | 41.36 | 3 |
| Carvacrol methyl ether | 32.86 | 2.4 |
| α-Terpinene | 21.12 | 2.2 |
| Spathulenol | 47.96 | 2 |
| Linalool | 25.59 | 1.8 |
| β-Myrcene | 19.60 | 1.5 |
| Total | - | 80.3 |
Compounds of Zataria multiflora
3.3. How to Use
A 500 mg of dried and powdered ZM leaves were filled in capsules via Chinese oven and prepared for use. Afterwards, groups of ZMSuppl and CRT + ZMSuppl received 500 mg of ZM every day after breakfast, one capsule (500 mg) with 100 mL of water. Groups of CRT (85%) and Control also consumed 100 mL of water with placebo capsules (Empty Capsules) after breakfast.
Training protocol CRT protocol was in such a way that before CRT, first the subjects were familiarized with the environment and exercises for a week and then one repetition maximum (1RM) of given exercises was determined. Sessions were conducted using circuit exercises’ design principles as an upper-body exercise and lower-body exercises as well as multi-joint movements at the beginning of the movements. Exercises included: 1. Squat, 2. Chest press, 3. Leg press, 4. Standing military press, 5. Knee extension, 6. Seated cable rowing, 7. Knee curl, 8. Biceps curl, 9. standing calf raise, 10. Triceps press, 11. Back extension, and 12. Crunch; 1RM was calculated using Brzycki equation (Equation 1).
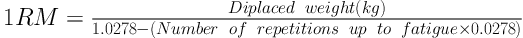
Subjects in CRT and CRT + ZMSuppl groups did the exercises with 85% of 1RM, at medium speed for eight weeks (three sessions per week). Each training session included five minutes warm-up and then doing 12 non-stop exercises between stations and duration of each station was 30 seconds. The number of repetitions at each station was recorded for the subjects. In each session, two sets (turn) of 12 exercises were carried out; between each turn, there was a three minutes active rest (30). Nutritional characteristics were recorded by subjects in the following two steps. All the subjects had a diet for two weeks. Subjects were advised to eat the same and specified food two days before blood sampling of pre-test and post-test (24 hours before the start of exercise or the first blood sampling, or pre-test and 24 hours after the end of exercise, the second blood sampling, or pre-test). The amount of nutrients received by subjects was calculated in all groups using the method described by McCance (31) (Table 3).
| Groups | Control | ZMSuppl | CRT | CRT + ZMSuppl | ||||
|---|---|---|---|---|---|---|---|---|
| Pre Training | Post Training | Pre Training | Post Training | Pre Training | Post Training | Pre Training | Post Training | |
| Total calorie, kcal/d | 2225 ± 190 | 2396 ± 103 | 2203 ± 157 | 2317 ± 98 | 2142 ± 125 | 2276 ± 180 | 2096 ± 113 | 2162 ± 125 |
| Total protein, g/d | 111 ± 16 | 112 ± 17 | 112 ± 14 | 110 ± 18 | 110.5 ± 17 | 108 ± 19 | 104 ± 16 | 102 ± 19 |
| Protein, g/kgBW/d | 1.05 ± 0.45 | 1.2 ± 0.4 | 1.1 ± 0.4 | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.1 ± 0.1 | 1.1 ± 0.2 | 1.1 ± 0.2 |
| Total protein, % energy | 3.18 ± 4.1 | 19.6 ± 3.5 | 18.4 ± 4.2 | 19.7 ± 4.9 | 19.5 ± 4.6 | 19.0 ± 4.2 | 20.9 ± 4.8 | 19.3 ± 3.8 |
| Total Carbohydrate, g/d | 295 ± 19 | 304 ± 23 | 285 ± 20 | 289 ± 25 | 248 ± 18 | 250 ± 24 | 252 ± 17 | 245 ± 22 |
| Total carbohydrate, % energy | 48.5 ± 6.9 | 50.8 ± 7.1 | 47.4 ± 7.2 | 50.5 ± 7.0 | 45.5 ± 9.8 | 49.0 ± 7.2 | 45.7 ± 10.1 | 49.3 ± 7.3 |
| Total fat g/d | 81.5 ± 21 | 77.3 ± 27 | 83.5 ± 23 | 78.0 ± 26 | 77.0 ± 22 | 71.0 ± 25 | 70.0 ± 17 | 66.0 ± 19 |
| Total fat, % energy | 31.9 ± 7.6 | 28.8 ± 6.9 | 32.5 ± 6.9 | 29.0 ± 6.4 | 32.6 ± 7.0 | 31.4 ± 6.5 | 31.8 ± 9.2 | 30.1 ± 6.3 |
Analysis of Received Nutrienta
For blood sampling, the following requirements were needed: 1. Non use of drugs and/or supplement during the study, 2. No change in diet at least two days before the test, 3. Lack of exercise other than the exercises of the study and a long walk at least 72 hours before the test, 4. Not drinking coffee, dark tea, bananas, cereal and heavy or greasy foods at least 24 hours before the test.
In two steps of 48 hours before the start of training and 48 hours after the last training session blood samples were taken after 12 hours of overnight fasting from a vein in the arm in a sitting position and poured in test tubes containing ethylenediaminetetraacetic acid (EDTA) and tubes without anticoagulant; samples were centrifuged at 3000 rpm for 10 minutes and, plasma that was used for the analysis of RBP-4 and TNF-α was separated. RBP-4 and TNF-α of plasma were measured using kits of Abcam Co., with Cat. No. ab108897 and ab46087, with sensitivity of 6 ng/mL and 10 pg/mL by ELISA method, respectively.
3.4. Statistical Analysis
The Kolmogorov-Smirnov and Leven tests were used to determine normality of data and homogeneity of variances, respectively. To compare data of the four groups, analysis of covariance (ANCOVA) was used. Alpha was considered at the level of 0.05. All data were analyzed using software SPSS version 22.
4. Results
Kolmogorov-Smirnov test showed that data distribution was normal (P > 0.05) and also Leven test showed that variances were homogenous (P > 0.05).
ANCOVA revealed that plasma levels of RBP-4 changed significantly in adaptation to CRT and ZMSuppl (f = 19.217, P = 0.001). Therefore, based on pairwise comparisons, plasma RBP-4 did not significantly change with ZMSuppl (P = 1.000); but, decreased significantly after eight weeks of CRT (P = 0.001), CRT + ZMSuppl (P = 0.001); therefore, the differences of plasma RBP-4 changes between these groups with ZMSuppl were significant ((P = 0.001) and (P = 0.001), respectively) (Figure 1).
ANCOVA revealed that plasma levels of TNF-α changed significantly in adaptation to CRT and ZMSuppl (f = 4.884, P = 0.005). Therefore, plasma TNF-α did not significantly change with ZMSuppl (P = 0.558) and CRT (P = 0.14); but, decreased significantly after eight weeks of CRT + ZMSuppl (P = 0.003); therefore, the differences of plasma TNF-α changes between this group with those of ZMSuppl and CRT were not significant ((P = 0.296) and (P = 0.988), respectively) (Figure 2).
5. Discussion
The findings of the current study showed that both eight weeks of CRT with 85% of 1RM and CRT + ZMSuppl in postmenopausal females in comparison to the control and alone ZMSuppl groups caused significant decrement in plasma RBP-4. Increased plasma values of RBP-4 are introduced as one of the factors affecting glucose intolerance disorder and diabetes in human and animal samples. The results of research shows that exercise training in untrained people and those with impaired glucose tolerance and diabetes caused reduction in RBP-4 concentration and weight (16). Conflicting results are reported on changes in plasma levels of RBP-4 after doing different training programs. Suri et al. also showed that eight weeks of alternative aerobic training (70% - 85% of heart rate (HR)max) causes significant reduction in the serum RBP-4 in patients with type 2 diabetes; also, a significant relationship was observed between baseline levels and changes in RBP-4. In addition, low levels of RBP-4 and triglycerides were associated with improved insulin resistance and increased insulin sensitivity (32). Graham et al. showed that four weeks of aerobic endurance exercise resulted in a significant decrement in plasma RBP-4 and insulin sensitivity increase in subjects with diabetes (33). But Choi et al. showed that 12 weeks of combined aerobic and RT (60% -75% of HRmax and 50% - 70% of 1RM) did not lead to significant changes in plasma RBP-4 in females with obesity (34). It seems that changes in plasma RBP-4 (decrement or no change) in adaptation to training is closely related to changes in weight and fat percentage (32-34), BMI, waist to hip ratio (34), and lipid profile (32-34). In the current study, also, subjects’ levels of lipid profile, body composition and insulin sensitivity were measured; however, due to being used in other reports, the values were not reported and can be subject to changes. Changes observed in insulin sensitivity are introduced as the main factor affecting RBP-4 changes. Graham et al. in another study showed that RBP-4 values through doing exercises significantly reduced only in patients with high insulin resistance, that is, a significant relationship was observed between the values of RBP-4 and insulin resistance index [the homeostatic model assessment (HOMA)-IR] (33). In another study, no significant relationship was observed between RBP-4 and insulin resistance index (HOMA-IR). The researchers believed that RBP-4 was not a proper index to determine insulin resistance (35). However, in another study by Cho et al. (36), the importance of RBP-4 was noted as a marker of insulin resistance and factor affecting secreting pancreatic beta cells. The researchers considered lack of a significant relationship between RBP-4 values and insulin resistance index (HOMA-IR) in relation to the small sample size. In general, the role of exercise in increasing insulin function by reducing the accumulation of intracellular triglyceride, increased fatty acid oxidation and mitochondrial biogenesis is well established (37).
But, on the other hand, the results of the current study showed a significant reduction in plasma levels of TNF-α in postmenopausal females only in CRT + ZMSuppl group compared to the control group. In other words, TNF-α was not affected by ZMSuppl alone and/or CRT alone; but, the cumulative effect of ZMSuppl and CRT could affect it. TNF-α is a complex cytokine that results in inhibition of lipoprotein lipase and stimulates lipolysis in adipocytes and increases unsaturated fatty acids in blood flow in such a way that the outcome is increased insulin resistance and diabetes (38). It is expressed more in lipid tissue and muscles of people with obesity and may inhibit insulin act in other tissues (37). However, it remains unclear, despite low concentrations of TNF-α, whether serum TNF-α inhibits insulin act. It is thought that TNF-α produced through fatty tissue locally affect insulin act by autocrine or paracrine methods and then secrete into the serum. Thus, increased production of TNF-α in lipid tissue may be the result of increased levels of TNF-α in blood circulation (38). Exercise can directly reduce cytokines’ production in lipid tissue, muscle, and mononuclear cells and indirectly increase insulin sensitivity and improve endothelial function. It is shown that acute and short-term exercise may be associated with increased levels of inflammatory adipocytokines, but doing a variety of regular exercises reduce circulating levels of inflammatory markers, even in the thin people (39). Training causes protection against insulin resistance caused by TNF-α. It also leads to reduced levels of TNF-α and IL-6 and increased levels of anti-inflammatory substances, such as IL-4 and IL-10 (40). Reduced TNF-α as a result of training may also be applied by IL-6-dependent and non-dependent paths (41, 42). In addition, training increases the levels of epinephrine and finally inhibits and reduces TNF-α response (43). Weight loss caused by doing training leads to reduced volume and the number of adipocytes and reduces the number of macrophages and endothelial cells. Increased production of anti-inflammatory mediators by adipocytes and production of hepatic fibrinogen and other pre-inflammatory mediators are other results of weight loss caused by training. However, the mechanism of the effect of circuit resistance training on reducing inflammation is not known well. It seems that intensive resistance training results in a significant reduction in plasma levels of TNF-α, even if no tangible and significant changes are observed in body composition and body fat mass. Results of a study showed that doing a combination of aerobic and resistance training with high intensity for 12 months as compared to aerobic training reduced the values of TNF-α more (44). This variable is independently associated with changes in fasting insulin. This relationship may be associated with TNF-α act and/or the production in muscles, since changes in TNF-α is positively associated with changes in body mass with no fat. On the other hand, studies showed that the transition from pre-menopause to menopause increases abdominal fat independent of the effects of age and total body fat. Given that obesity (due to the production and expression of pro-inflammatory cytokine gene of TNF-α) is a factor associated with high inflammation levels (11, 45), therefore, reducing body fat and increasing lipolysis due to physical activity is a mechanism that reduces inflammation. An important feature of the current study was that the weight and body mass of subjects were the same as before the start of the study. This separates the effect of training from the effect of the weight and body mass. Also, it is shown, that the relationship between more physical activity and physical fitness and less inflammation is independent of obesity (17). Thus, according to the current study matched subjects, it seems that there are other mechanisms by which physical activities reduce inflammation.
In addition to the positive role of resistance training on the reduction of inflammatory factors, such as reducing the RBP-4 in CRT, when ZM intervention was added to the study (CRT + ZMSuppl), it reduced plasma RBP-4 and also TNF-α.
ZM has several compounds (such as thymol and carvacrol). The cumulative effects are attributed to these compounds that can prevent fat deposit in vessels (46). Also, ZM reduces the levels of blood triglycerides due to possessing terpenoids and flavonoids compounds. In addition, ZM has a very high antioxidant feature, which in addition to reducing blood fat can inhibit low density lipoprotein (LDL) (28). Therefore, considering the significant relationship between RBP-4 blood triglycerides, it seems that ZM can also reduceRBP-4 by reducing blood triglyceride. In the past two decades, ZM’s anti-inflammatory and oxidative anti-stress effects are reported (47). Lee and Shibamoto proved the antioxidant effects of ZM (48). Dorman et al. indicated that one of the best sources of natural antioxidants is phenolic compounds in the herbal essentials, especially ZM (49). Recent studies showed that phenolic compounds in ZM and its derivatives are responsible for its antioxidant activity, which lead to a significant reduction in serum LDL levels and markers of oxidative stress (50). Sabu et al. showed that polyphenols available in the plant inhibit lipid peroxidation, radicals’ superoxide, and reduces serum glucose levels in diabetic rats. Polyphenols available in green tea increase the antioxidant capacity (51). Rana and Soni found that ZM improves antioxidant potential and thus prevents oxidative stress (52). Joekim et al. reported that the effect of cyclooxygenase enzymes and oxidative stress on changes during development and cell differentiation such as a variety of cancers was proved and it was found out that in ZM, there are compounds that inhibit the mentioned enzymes and/or their antioxidant effects reduce oxidative stress (53). Also, Diaz-Cruz et al. found that cyclooxygenase through prostaglandin E2 production increases aromatase enzyme that can convert androgens to estrogen. On the other hand, compounds available in Zataria sp. by aromatase inhibition by cyclooxygenase inhibitors reduce estrogen level, which also in turn leads to a reduction in oxidative stress and inflammatory factors (54). Therefore, regarding low levels of RBP-4 and TNF-α, in the present study, it seems that CRT + ZMSuppl can be effective in reducing inflammatory and injury-causing factors and the prevention and treatment of a variety of chronic inflammatory diseases, such as cardiovascular diseases and diabetes. According to the total presented features of ZM, it can be concluded that perhaps low levels of RBP-4 and TNF-α in the present study were due to the presence of compounds such as glucuronic acid, phenolic compounds and their derivatives, flavonoids, antioxidant features, and other substances available in ZM.
5.1. Conclusions
The findings of the current study showed the positive effect of CRT and ZMSuppl on plasma RBP-4 and TNF-α in postmenopausal females. Therefore, it is likely that regular CRT and consuming traditional herbal medicines such as ZM is a good plan to decrease abnormalities related to insulin, glucose resistance, and inflammatory factors resulting from menopause. The strength of the current study was the type of training that had a kind of impact on adipose tissue and great pressure on heart compared to traditional RT; besides, it determined that ZM combinations with GC-MS, and received food record from participants. But, the weakness was lack of reporting lipid profile and insulin resistance related to RBP-4 changes; due to reports in the other manuscript by other colleagues. The limitations of the current study were lack of control over participants’ motivational condition and access to them when out of training; and also participants’ latent diseases.