1. Background
Leishmaniasis is one of the oldest zoonotic diseases faced by humans since 650 before Christ (BC). This commonplace infection is caused by a kinetoplastid parasite, “Leishmania” (family Trypanosomatidae), with a wide range of manifestations, from painless lesions or severe cutaneous wounds (due to Leishmania major) to visceral form (owing to Leishmania donovani) (1). The female sand flies of the genera Lutzomyia (in the new world) and Phlebotomus (in the old world) are the main vectors of Leishmaniasis, which transfer the promastigote form to the susceptible host. Nonetheless, this infection remains to be an important public health issue and has remarkably drawn attention of hygiene surveillance systems to take necessary measures. Two genera of Leishmania render the cutaneous form in Iran, namely Leishmania tropica and L. major. The annual morbidity and mortality rate of cutaneous leishmaniasis (CL) are 1.5 million cases and 7000 deaths, respectively. The incidence rate of CL is increasing annually, because of co-incidence with immune deficiency diseases such as human immunodeficiency virus (HIV), international travel as well as refugees coming from endemic regions (2, 3).
Chemotherapy and immunoprophylaxis of Leishmaniasis are of utmost importance (4). Due to its long-term therapy, treatment of Leishmaniasis has become a great challenge. The principal first-line therapies to treat CL are pentavalent antimonial compounds such as sodium stibogluconate or Pentostam and meglumine antimoniate, whereas the second-line therapies are Pentamidine and Amphotericin B. However, these medications are expensive with low availability, undesired side effects (nausea, vomiting) and relapse while drug resistance has been reported after their use (5). Hence, there is an urgent need to find novel drugs and compounds, which possess minimum complications, and are available and economical. However, many researches have been performed assessing the effect of synthetic and/or natural products on these parasites to discriminate their leishmanicidal activity, but they have had different efficacies and, so far, there isn’t any novel therapy available. According to the World health organization (WHO) publications, plants are among the best and the largest repositories of biomedical compounds for humans; therefore, evaluation of herbal extracts could lead us to cutting-edge treatment of cutaneous leishmaniasis (6). Chaerophyllum (family Apiaceae) consists of 110 species of fragrant shrubs, of which eight species exist in Iran and grow predominantly in North temperate areas. Chaerophyllum macropodum Boiss is a known and characterized species, growing in many geographical territories, with its aerial parts being used occasionally as edible vegetable and in preparation of foods and cheese in Iran and Turkey (7-10).
2. Objectives
Given that this plant has been implicated in anti-bacterial experiments against some bacterial strains (7, 11, 12) as well as on Trichomonas vaginalis (13), yet its anti-leishmania properties has not been identified; thus we aimed to elucidate the anti-leishmanial activity of Chaerophyllum against promastigotes of L. major.
3. Methods
3.1. Preparation of Ethanolic Extract
Aerial parts of C. macropodium were collected from Roudbarak, which is located 65 km North East of Mahdishahr, Semnan, Iran. Plants were identified by experienced botanists at the University of Applied Science and Technology (UAST), Semnan branch. A voucher specimen of C. macropodium has been deposited in the herbarium of medicinal plants research UAST. Plant leaves were washed with running tap water. All parts of the plants were dried in the shade for a week and then crushed into small pieces and finally powdered using an electric blender. Plant leaves powder was then stored in plastic bags for further utilization. Plant extracts were obtained by the following procedures: ethanolic extract was obtained by incubating 50 g of powdered leaves with 500 mL of ethyl acetate: methanol: distilled water (6:3: 1) overnight at room temperature before adjustment to 40°C for eight hours in a soxhlet system. In all cases, the extracts were centrifuged (3000 rpm) for 15 minutes and the supernatants were harvested and filtered using Whatman paper No. 1. The solvents were evaporated by incubation at room temperature. The extraction yields were calculated as a percentage of the used powder (14).
3.2. Parasite Culture
Leishmania major parasites (MRHO/IR/75/ER) were obtained from Razi vaccine and serum research institute of Iran. The promastigotes were cultivated in Roswell Park Memorial Institute (RPMI) 1640 medium containing 100 units/mL penicillin, 100 μg/mL streptomycin and 20% fetal bovine serum (FBS) in a 25 ± 1°C incubator.
3.3. Promastigote Assay
Sterile 96-well plates were prepared. Subsequently, a volume of 100 µL of promastigotes (106 parasites/mL) was added to every well. As a control well, lacking any drug, 100 µL of drug solvent was added. Furthermore, 100 µL of solutions containing the plant extract with different concentrations (6.25, 12.5, 25, 50, 100 and 200 µg/mL) were added to each well; thus to adjust to a final amount of 200 µL, 100 µL of promastigotes were added to every well. It is noteworthy to mention that all of these experiments were carried out in triplicates and three plates were incubated at 22 - 25°C. The number of promastigotes was calculated after 24, 48 and 72 hours by a hemocytometer slide. The IC50 of Chaerophyllum was calculated after 24 hours by Graph Pad Prism 5 software.
Promastigote and J774 viability assay by microculture tetrazolium test (MTT) [3- (4, 5 dimethylthiazol-2-yl) - 2, 5-diphenyltetrazolium bromide]: The leishmanicidal characteristics of Chaerophyllum plant extract on promastigote stage was assessed in vitro. In 96-well microtiter plates, 106 promastigotes of Leishmania major (in logarithmic phase) and J774 macrophages per well were cultured in RPMI 1640 (Gibco) and 20% FBS (Gibco) and allowed to multiply for 24 hours in the medium alone (negative control group), in solvent (the other control group) and in the presence of Chaerophyllum extract, with 6.25, 12.5, 25, 50, 100 and 200 µg/mL concentrations. Promastigotes and macrophages were incubated at 24°C and 37°C, respectively. Thereafter, 20 µL of tetrazolium (Roche, Germany) (5 mg/mL) was added to each well and plates were incubated for four hours. Afterwards, they were centrifuged at 1000 g for 10 minutes, the supernatant was discarded and each well was filled with 100 µL of dimethyl sulfoxide (DMSO), re-suspending the contents. At last, the optical density (OD) of every well was read by the enzyme linked immunosorbent assay (ELISA) reader at 570 nm. Percentage of viability was calculated by:

Flow cytometry analysis for inducing apoptosis: Flow cytometry was based on the principle in which a laser beam is emitted toward a cell population and refracts. Study of the diffraction of the resulting light in different directions tells us some information about the size, shape and structure of the cell. In order to discern the type of cell death, i.e. necrosis or apoptosis, for J774 macrophages challenged with various concentrations of Chaerophyllum, Annexin V and Propidium iodide (PI), staining was used. In this research, the Biovision kit (Palo Alto, Califor-nia, USA) was used. The challenged cells with different concentrations of Chaerophyllum extract and the cells of the control group were washed and centrifuged at 1400 g for one minute. According to the manufacturer’s protocol, 500 µL of binding buffer, 5 λ Annexin and 5 λ PI were added. Finally, the samples were incubated in a dark room for five minutes. The color density of cell-absorbed Annexin V was assessed by FACSCanto II. The obtained results were analyzed with the FlowJo software.
3.4. Data Analysis
In order to analyze the obtained results, we used one-way analysis of variance (ANOVA) test (SPSS software version 20) and a probability value (P value) of < 0.05 was considered as statistically significant.
4. Results
4.1. Anti-Promastigote Assay
After challenging promastigotes with different concentrations of the Chaerophyllum extract (6.25, 12.5, 25, 50, 100 and 200 µg/mL) in three separate microtiter plates, the inhibition concentration (IC50) for promastigotes was read during 24, 48 and 72 hours, in triplicates (Table 1); pertinent to these results, the difference between challenged and control groups was statistically significant with one-way variance analysis test (P < 0.05). The IC50 of Chaerophyllum extract was 80.62 µg/mL after 24 hours.
| Conc. / Period | 6.25 µg/mL | 12.5 µg/mL | 25 µg/mL | 50 µg/mL | 100 µg/mL | 200 µg/mL | Control |
|---|---|---|---|---|---|---|---|
| 24 hours | 672 ± 22.62 | 632 ± 11.31 | 576 ± 0 | 552 ± 11.31 | 488 ± 11.31 | 88 ± 11.31 | 720 ± 22.62 |
| 48 hours | 648 ± 33.94 | 600 ± 33.94 | 536 ± 11.31 | 472 ± 11.31 | 432 ± 22.62 | 88 ± 11.31 | 760 ± 11.3 |
| 72 hours | 648 ± 11.31 | 584 ± 11.31 | 440 ± 33.94 | 312 ± 11.31 | 218 ± 37.47 | 64 ± 0 | 800 ± 00 |
Mean ± Standard Deviation of Promastigotes (× 106) Treated With Different Concentrations of Chaerophyllum Extract (6.25-200 µg/mL)
4.2. Promastigote Viability Assay by MTT
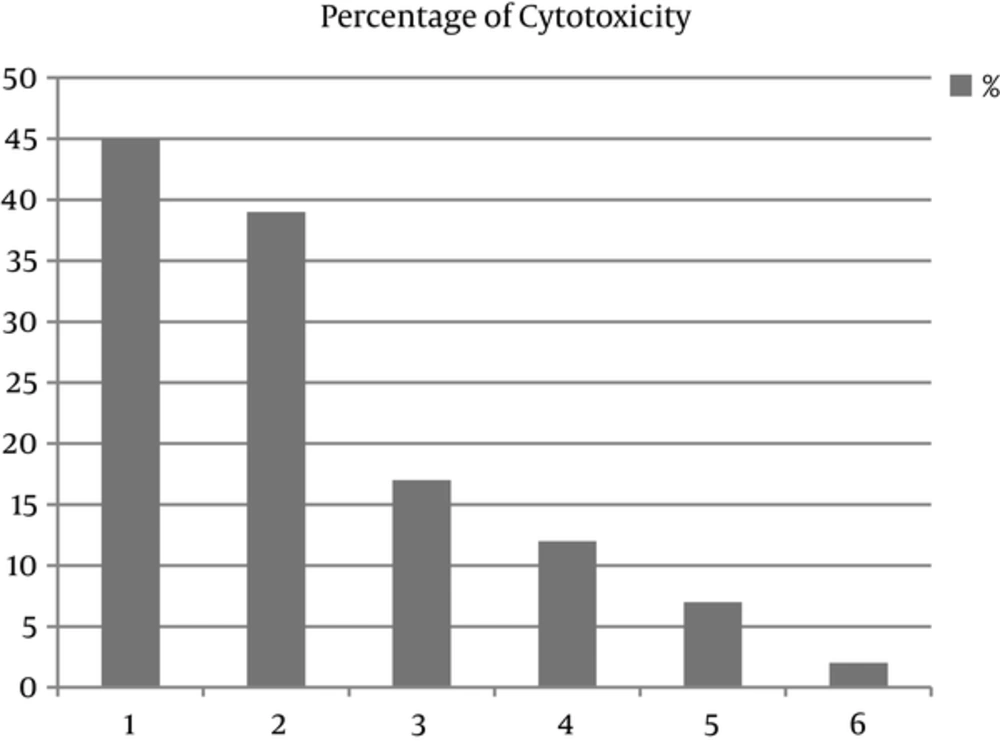
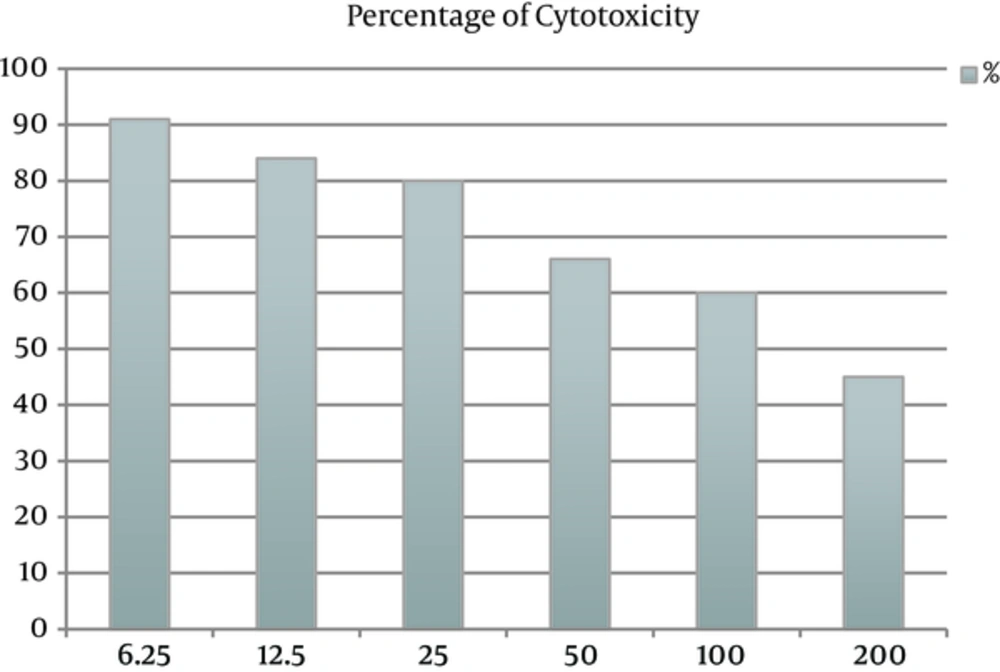
The cytotoxic effect of Chaerophyllum extract on promastigotes of L. major and J774 macrophages was evaluated at six concentrations (6.25, 12.5, 25, 50, 100 and 200 µg/mL) for 24 hours. The concentration of 200 µg/mL of Chaerophyllum after 24 hours indicated maximum cytotoxic effect on both promastigotes of L. major and J774 macrophage. The results showed that by increasing the Chaerophyllum concentration, the viability of promastigotes decreased. The percentage of cytotoxicity of promastigotes and J774 macrophages after adding Chaerophyllum extract was 91% and 45%, respectively (Figures 1 and 2).
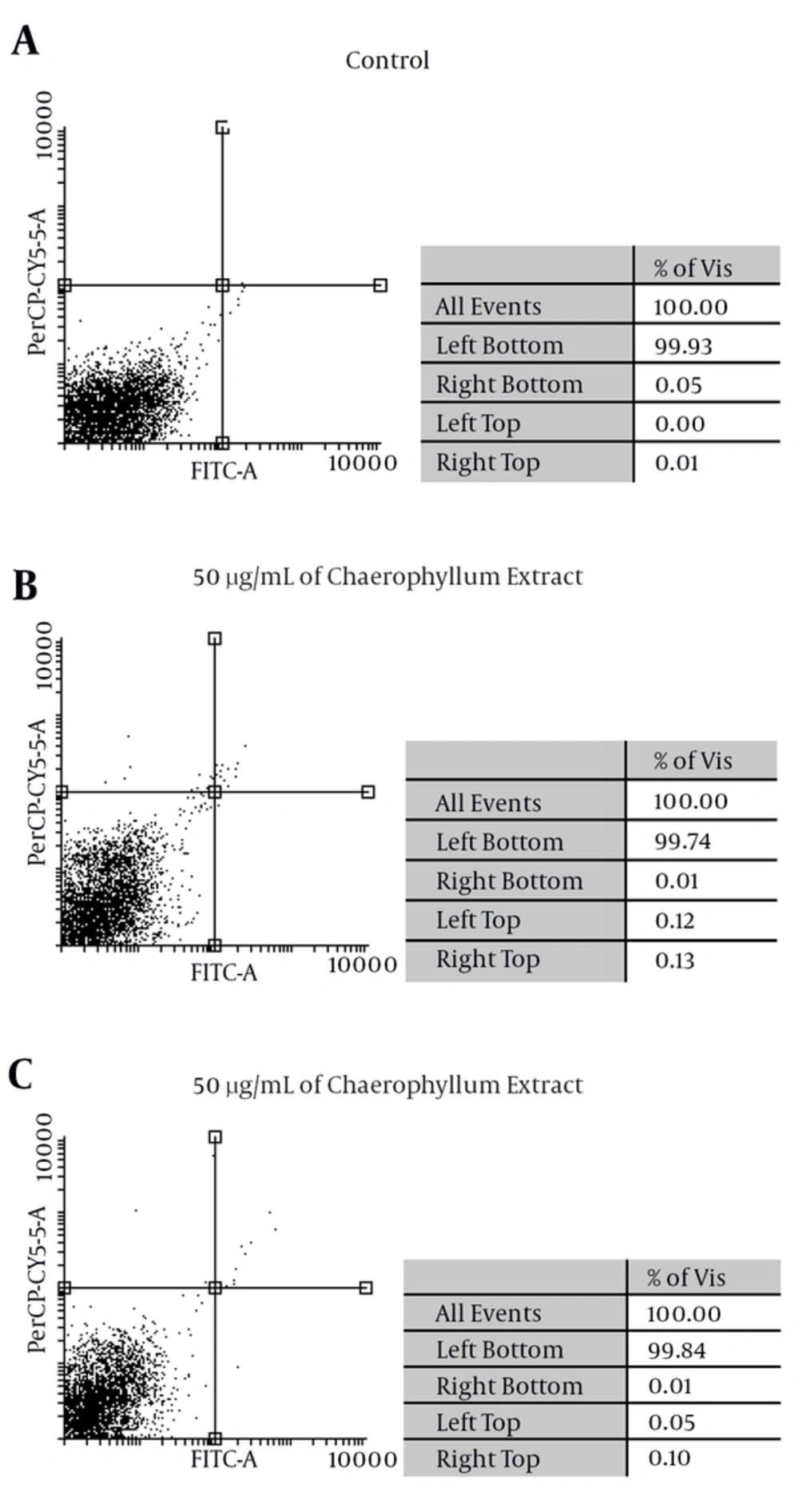
Induced apoptosis by Chaerophyllum: Flow cytometric analysis suggests, after adding Chaerophyllum extracts to the J744 macrophage with two concentrations (50 and 100 μg/mL) of Chaerophyllum extract for 24 hours, necrotic and apoptotic effects in the J774 macrophages emerge. The percentage of apoptosis (early and late apoptosis) in macrophages induced by 50 and 100 μg/mL of Chaerophyllum extract after 24 hours was 0.01%, 0.13%, 0.01% and 0.1%, respectively (Figure 3)
5. Discussion
Leishmaniasis, one of the most outstanding infectious diseases in the world, has various clinical manifestations. Cutaneous leishmaniasis (CL) is a self-limiting complication while visceral disease is fatal and often leads to death. There is a gap in the field of CL treatment, as the drugs of choice are expensive, have side effects and a serious problem being “drug resistance” may develop in the parasites. Hence, alternative medicines and novel compounds are of great interest. There is a vast and potential source for new and selective agents for the treatment and prophylaxis of many infectious diseases such as CL (15). In compliance with WHO, up to 80% of the world’s population depend on traditional medicine for their primary health care requirements. In the past decades, the development of combinatorial antimicrobial agents has entailed reluctance in herbal medicine as an opulent supply for antimicrobial agents (16). However, in the recent years, some limitations have evolved in case of synthetic biochemical agents, which have pointed out scientists to the herbal medicine research field (17). In this sense, the potential of plant extracts as a source of anti-leishmanial remedies has been demonstrated. Multiple papers have evaluated and reported the anti-Leishmania characteristics of plant extracts and their bioactive ingredients such terpenic derivatives, carvacrol, p-cymene, thymol, carvone, limonene and terpinene (18-25).
In the present investigation, the in vitro anti leishmanial attributes of alcoholic extract of chaerophyllum were assessed against L. major. Our results showed that Chaerophyllum have dose-dependent anti leishmanial properties. The number of parasites showed a statistically significant difference between sample and control groups. We demonstrated that alcoholic extract of Chaerophyllum plant could inhibit the proliferation of promastigotes at various doses (6.25, 12.5, 25, 50, 100 and 200 µg/mL) at 24 hours of culture (P < 0.05). The obtained data from optical density (OD) and IC50 showed that concentration of 80.62 µg/mL of alcoholic extract of Chaerophyllum had an inhibitory impact on promastigotes of L. major. During the MTT procedure, formazan dye was the reason of elevated levels of optical absorbance, which was produced by the metabolic activity of the mitochondrial enzyme, dehydrogenase, in viable cells and it is correlated with the number of alive cells.
These findings were in agreement with the results obtained from previous studies, suggesting the anti-leishmanial features of frequently utilized plants, indicating their potential to be used as novel anti-infectious agents. Ebrahimisadr et al. (2012) (26) showed the anti-promastigote properties of Artemether drug with an IC50 of 25 µg/mL. In the investigation of Ezatpour et al. (2014) (27) on Pistacia khinjuk extract against L. major, it was shown that the growth of promastigotes was inhibited and IC50 of the plant extract was 58.6 ± 3.2 µg/mL. In a study conducted by Shemshadi et al. (2015) (28), the effect of Capparis spinosa extract was evaluated on promastigotes of Leishmania major and similar results were obtained in terms of macrophage inhibition, as compared to glucantime. In a study conducted by Yousefi et al. (29), various concentrations of Saffron were appraised on promastigotes and amastigotes of L. major in a time-dependent manner; they showed that extract IC50 was 0.7 mg/mL after 48 hours.
The main goal of our study was to investigate the effects of various concentrations of Chaerophyllum extract on Leishmania major promastigotes; also, this extract was evaluated on J774 macrophage cell line to ensure it does not have any harmful effect on them. Finally, the discoveries of our study asserted that Chaerophyllum alcoholic extract possesses strong anti-leishmanial properties and additionally we recommend it to be examined on amastigotes of L. major. These findings also provided scientific and convincing evidence that plants and their natural compounds could be applicable in traditional medicine for the cure and prevention of cutaneous leishmaniasis, especially in low-income countries having endemic conditions in terms of CL. As the preparation of vaccines needs high technology and is expensive while these vaccines provide partial protection, thus herbal drugs with low toxicity should be considered (29).