1. Background
Uremic pruritus (UP) is a common, bothersome symptom in hemodialysis (HD) patients with end-stage renal disease (ESRD) (1, 2). UP is associated with poor physical conditions and a sizeable decline in quality of life (3, 4). The prevalence of UP is variable; for example, its prevalence is 38% in France but 55% in Italy (5). According to one study in Iran, the percentage of patients with UP is 41.9% (6).
The underlying pathophysiological mechanism of UP is poorly determined; consequently, no effective treatment currently exists. Multiple factors have been suggested to play a role in the occurrence of UP, including xerosis, hyperphosphatemia, hyperphosphaetemia, secondary hyperparathyroidism, the stimulation of opioid receptors, iron deficiency anemia, the proliferation of histamine-secreting skin mast cells, and generalized inflammation (7-9). Immunohypothesis is currently favored as an explanation of the cause of UP (10). Some studies have suggested that UP is related to the involvement of immune system with the derangement of T helper (Th) cells differentiation (more have nominated Th1 differentiation as the responsible parameter) (11).
The balance between Th1 and Th2 subsets distinguishes the immune status. In a Th1-dominant state, where interferon-γ (IFN-γ) and interleukin (IL)-2 are produced, an inflammatory response is presented. In contrast, Th2 cells produce anti-inflammatory cytokines like IL-4 and IL-5 that inhibit Th1 activation (12). The cytokines of Th1 and Th2 cells have negative effects on the function and growth of each other (13). One study indicated that the IL-2 mean serum level in HD patients with UP is higher compared with those without UP. This difference implies that HD patients with UP have an overactivity of Th1 (14). In another study, turmeric decreased UP and high-sensitivity C-reactive protein (HS-CRP), possibly because of its anti-inflammatory properties (15).
The aerial part of Fumaria parviflora L (FP, Fumariaceae), named Shah’tareh in Iran, has been mentioned as a treatment of itching in traditional Persian medicine sources, such as Canon of Medicine by Ibn-e-Sina, or Avicenna (980 - 1037 AD) (16, 17), and The Storehouse of Medicaments by Aghili (written in 1772 AD) (18, 19). In vivo and in vitro studies have shown that Shah’tareh has hypoglycemic (20), laxative, prokinetic (21), antidiarrhea, bronchodilative, antispasmodic (22), and hepatoprotective (23) pharmacological effects. Shah’tareh has also been considered to be useful for healing eczema (24). This medicinal plant contains several alkaloids and organic acids. Fumaric acid is one of its most efficacious components (25, 26). Moreover, FP contains fumaric acid esters (FAEs) in addition to monomethylfumarate (MMF), the most bioactive metabolite of FAEs (24, 27). FAEs have anti-inflammatory effects; they can block the proinflammatory actions of human C-reactive protein (CRP) (28). Some studies showed that MMF can increase IL-4, an anti-inflammatory interleukin, or can decrease IFN-γ, an inflammatory factor. These effects can reduce inflammatory responses (13, 29).
2. Objectives
Based on the findings of previous studies, we designed this placebo-controlled clinical trial to evaluate the effects of FP on the reduction of UP. We hypothesized that FP well reduce the severity of UP via inflammatory response suppression.
3. Methods
3.1. Study Design
This is a randomized, double-blind, placebo-controlled trial with two parallel arms in order to establish a 1: 1 allocation ratio between the intervention and control groups.
3.2. Ethical Considerations
This study was approved by the ethics committee of Shiraz University of Medical Sciences (SUMS) and is registered with ID number IR.SUMS.REC.1394.63. Our trial is also recorded at the Iranian registry of clinical trials database (irct.ir) with study number IRCT2015080110410N2. All subjects were provided with written informed consent forms for participation in this study.
3.3. Plant Material
The FP was purchased from a herbal market in Shiraz in South of Iran. Taxonomic identification was confirmed by a botanist at the herbarium center of Shiraz school of pharmacy at SUMS to store a specimen voucher (PM682). Quality control tests, including microbial contamination, were based on the United States pharmacopeia’s guidelines (USP, 2006). FP powder was packed in 500-mg capsules. Wheat flour was chosen as a placebo, which was packed in the same type of capsules. A total of 42 capsules were packaged into each identical dark PET containers for a week-long period of use by patients.
3.4. Study Population
A total of 210 patients with ESRD who had been undergoing HD for at least six months at Abu-Ali-Sina dialysis center, the referral center for HD patients in Southern Iran, were screened for this present study, which was conducted between September 2015 and December 2015. Subjects were eligible for participation if (a) they were over 18 years old, (b) underwent HD two or three times a week, (c) were suffering from pruritus for at least six weeks, (d) were sufficiently dialyzed with a minimum single Kt/V of 1, and (e) had not improved with conventional drugs. Patients were excluded if they had hepatobiliary diseases, respiratory ailments, malignancy, allergic diathesis, or any dermatologic diseases that induce pruritus. Patients were also excluded upon receiving immunosuppressive therapy. Additionally, the visual analogue scale (VAS: a score of 0 represented the absence of pruritus and a score of 10 represented the most severe symptoms) was used to assess the patients’ symptoms, and patients with a VAS score lower than 4 were excluded.
3.5. Procedures
All medication with antipruritic effects was stopped one week prior to starting the treatment phase. Patients were randomly divided into two groups to receive either two FP capsules (with each capsule containing 500 mg FP powder) or two placebo capsules (containing wheat flour) three times a day 15 minutes before meals. Balanced blocked randomization with a block size of four was used. Each set of eight bottles were packed into one container, each of which was numbered for each patient. This arrangement was based on a randomization list. The patients received one bottle from their container each week. All the participants and the investigator were blinded to group assignment. Code-breaking was carried out after data analysis.
3.6. Efficacy Measures
The severity of each patient’s pruritus, which was based on their VAS score, was assessed during their HD session one week prior to the initiation of the study. The mean of these was deemed to be their baseline VAS scores, and the VAS was used to estimate the severity of the patients’ pruritus at the end of each week for eight weeks (the period of drug administration) and four weeks after the discontinuation of drug administration. During the study, all scores were recorded by a general physician. Furthermore, before study entry and at the end of each week during the study period (eight weeks), the patients answered the detailed pruritus questionnaire proposed by Duo (15), which calculated the score of their pruritus based on the severity, distribution, and frequency of pruritus-related sleep disturbances:
• Severity: A slight itching sensation with no need to scratch received 1 point. Itching with a slight need to scratch without excoriation received 2 points. An itch with a frequent need to scratch without excoriation received 3 points. Itching with scratching and excoriation received 4 points. Pruritus that led to total restlessness received 5 points.
• Distribution: Itching at a single area received 1 point, itching at two or more areas received 2 points, and generalized pruritus received 3 points.
• The scores for severity and distribution were multiplied separately for morning and afternoon then were then added together; the maximum score was 30.
• Sleep disturbances: Each episode of waking up because of pruritus received 2 points (maximum: 10 points), and each scratching episode accompanied by excoriation during the night received 1 point (maximum: 5 points).
• The final score was calculated by adding the sleep disturbances score to the severity-distribution score (maximum: 45 points).
In all patients, after a long dialysis-free weekend period and before HD, blood samples were drawn from the arms used for HD access. The samples were used to determine each patient’s serum IL-4 level, IFN-γ, HS-CRP, and parathyroid hormone (PTH), which were measured by ELISA kits (eBioscience, Vienna, Austria, Platinum ELISA kits for IL-4 and IFN-γ; BIOMERICA, USA, ELISA kits for HS-CRP and PTH), hematocrit (Hct), hemoglobin (Hb), white blood cells (WBC), alanine transaminase (ALT), aspartate transaminase (AST), albumin, calcium, phosphorus, blood urea nitrogen (BUN), and creatinine (Cr). The gathered blood samples were placed in an ice-box and were immediately transferred to the lab. To measure HS-CRP, IL-4, and IFN-γ, blood samples were immediately centrifuged and stored in a specific freezer in -80°C. Repeated sampling was drawn for IL-4, IFN-γ, HS-CRP, ALT, and AST after eight weeks of drug intake. Dialysis efficacy was calculated according to Daugirdas’s formula (30), time spent on HD treatments (months), and age (years).
3.7. Statistical Analysis
Statistical analysis was done using SPSS version 18. All continuous data was reported as a mean ± SD. An independent sample t-test was used for the comparison of continuous data between the two groups. Qualitative variables were reported as percentages, and a Chi-squared test was applied for comparison between the two groups. A paired-sample t-test was performed for the detection of the differences within groups. Correlations were computed using Spearman’s rank correlation. The level of significance was considered as P < 0.05.
4. Results
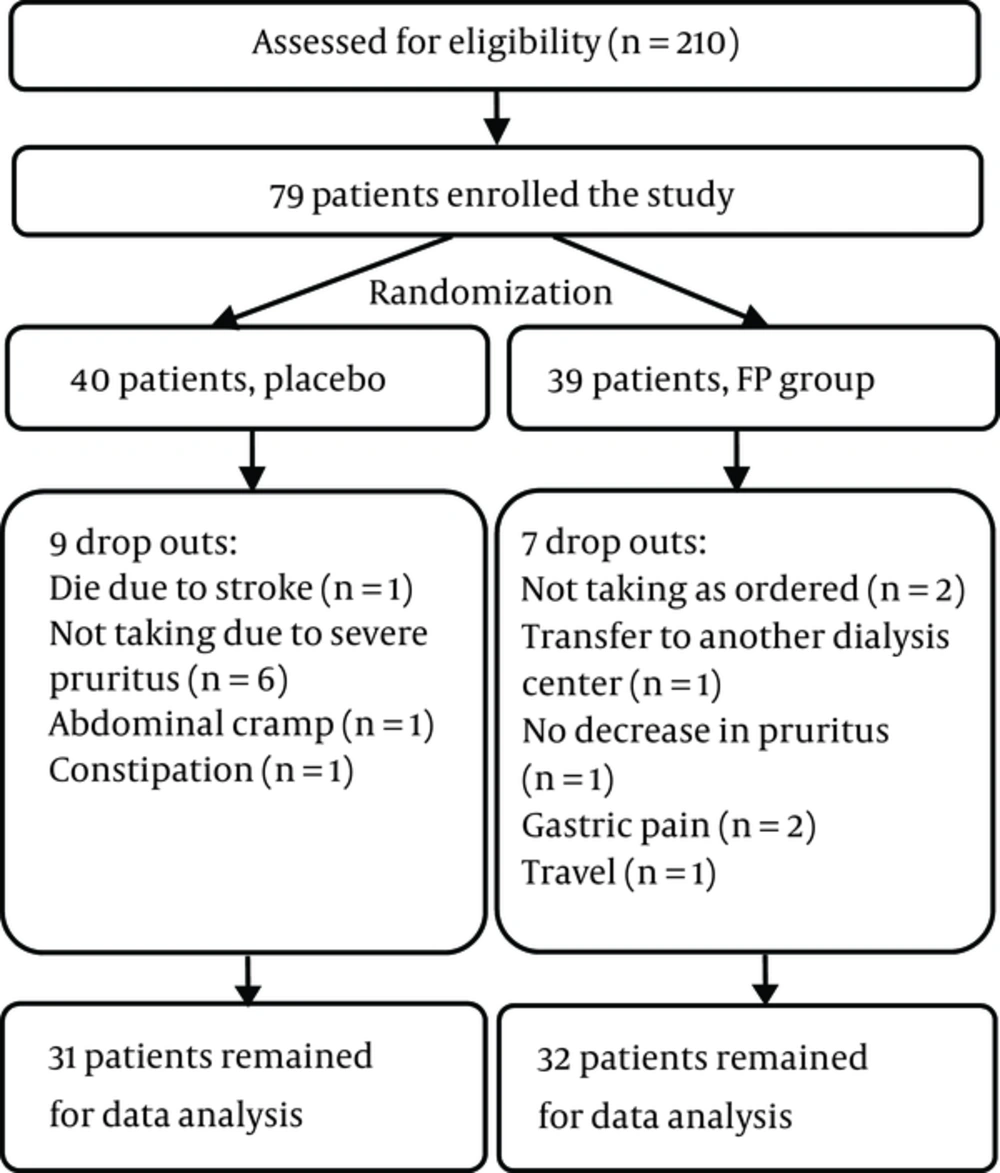
4.1. Study Flow and Baseline Subject Characteristics
Out of 210 dialysis patients in Abu-Ali-Sina Dialysis Center, 79 patients met this study’s inclusion criteria, were enrolled in the study, and were randomized into two groups. A flow diagram of the clinical study is shown in Figure 1. Data analysis was performed on 32 out of 39 patients in the FP group and on 31 out of 40 patients in the placebo group. The basic demographic and laboratory data of the patients are laid out in Tables 1 and 2, respectively. No significant differences in this data were detected between the two groups.
| Patient characteristics | Fumaria parviflora L. | Placebo | P Value |
|---|---|---|---|
| Number | 32 | 31 | |
| Age (years), mean ± SD | 53.5 ± 14.2 | 57.3 ± 13.4 | 0.284 |
| Sex (male), n (%) | 21 (65.6) | 19 (61.3) | 0.128 |
| Duration on HD (months), mean ± SD | 61.5 ± 52.5 | 55.2 ± 33.2 | 0.573 |
| Kt/V, mean ± SD | 1.43 ± 0.16 | 1.46 ± 0.16 | 0.579 |
| Number of dialysis per week, med (rang) | 3 (2 - 3) | 3 (2 - 3) | 0.290 |
| BMI, mean ± SD | 23.41 ± 4.41 | 22.20 ± 3.87 | 0.255 |
| Family history of renal failure, n (%) | 6 (18.8) | 6 (19.4) | 0.951 |
Abbreviations: SD, standard deviation; n, number; BMI, body mass index.
| Laboratory Parameter | Fumaria parviflora L. | Placebo | P Value |
|---|---|---|---|
| Hct (%) | 36.29 ± 4.67 | 36.31 ± 4.35 | 0.984 |
| Hb, g/dL | 11.65 ± 1.21 | 11.60 ± 1.39 | 0.880 |
| WBC, N/µL | 7046.87 ± 1840.75 | 6519.35 ± 2175.68 | 0.302 |
| AST, U/L | 15.21 ± 8.73 | 14.92 ± 14.91 | 0.934 |
| ALT, U/L | 16.30 ± 9.94 | 15.64 ± 9.99 | 0.812 |
| Albumin, g/dL | 3.68 ± 0.54 | 3.82 ± 0.23 | 0.195 |
| BUN, mg/dL | 51.06 ± 13.06 | 51.96 ± 13.62 | 0.789 |
| Cr, mg/dL | 8.78 ± 2.06 | 7.92 ± 2.12 | 0.109 |
| PTH, pg/mL | 380.87 ± 236.49 | 339.54 ± 188.02 | 0.447 |
| Calcium, mg/dL | 8.86 ± 0.71 | 8.94 ± 0.91 | 0.679 |
| Phosphorus, mg/dL | 5.03 ± 1.19 | 4.84 ± 0.99 | 0.485 |
| IL-4, pg/mL | 4.83 ± 2.72 | 9.73 ± 18.34 | 0.159 |
| IFN-γ, pg/mL | 4.93 ± 2.21 | 5.18 ± 2.02 | 0.637 |
| HS-CRP, mg/dL | 0.47 ± .22 | 0.52 ± 0.23 | 0.394 |
aValues are expressed as means ± SD; N, number.
4.2. Clinical Response
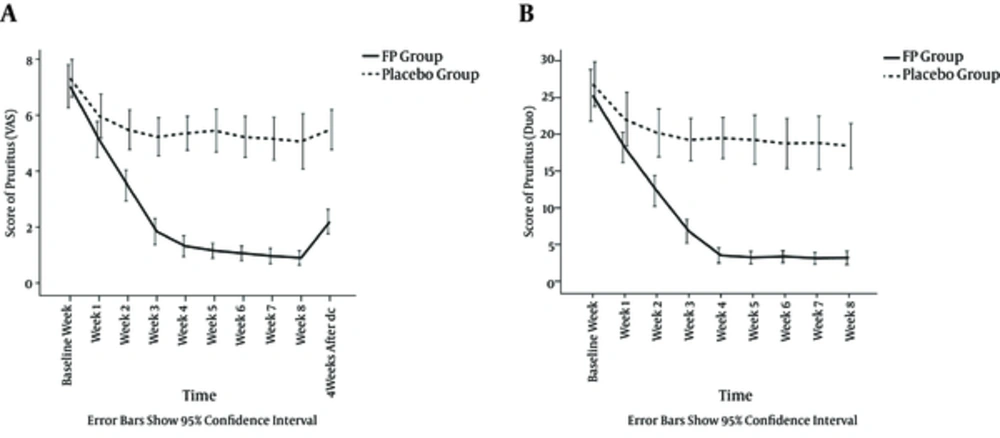
In order to assess each patient’s pruritus accurately, two different tests, the VAS and Duo, were used, the scores of which were significantly correlated at the baseline (r = 0.95, P < 0.001).
The mean VAS pruritus score was 7.03 ± 2.07 in the FP group, which declined to 0.88 ± 0.70 after eight weeks of treatment (P < 0.001). The Duo score was 25.13 ± 9.46, which decreased to 3.09 ± 2.55 after eight weeks of treatment (P < 0.001). In the placebo group, the mean VAS pruritus score was 7.32 ± 1.81, which fell to 5.06 ± 2.70 after eight weeks of treatment (P < 0.001). The initial Duo score was 26.81 ± 8.28, which decreased to 18.42 ± 8.33 after eight weeks of treatment (P < 0.001).
A repeated-measures analysis of variance showed that there were no significant differences between the baseline VAS (P = 0.554) and Duo (P = 0.456) scores of the groups. However, during the study, the VAS and Duo scores were considerably different between two groups each week’s assessment (P < 0.001) except the first week (P = 0.06). The severity of pruritus during the period of study in both groups is shown in Figure 2.
In the FP group, the decrease in pruritus score was significant from baseline to week 1, week 1 to 2, week 2 to 3, and week 3 to 4 (all P < 0.001). Nevertheless, the decrease was not significant after week 4. Four weeks after the discontinuation of the drug, the VAS scores in the FP group and the placebo group were 2.16 ± 1.19 and 5.48 ± 1.94, respectively (P < 0.001).
4.3. Biochemical Response
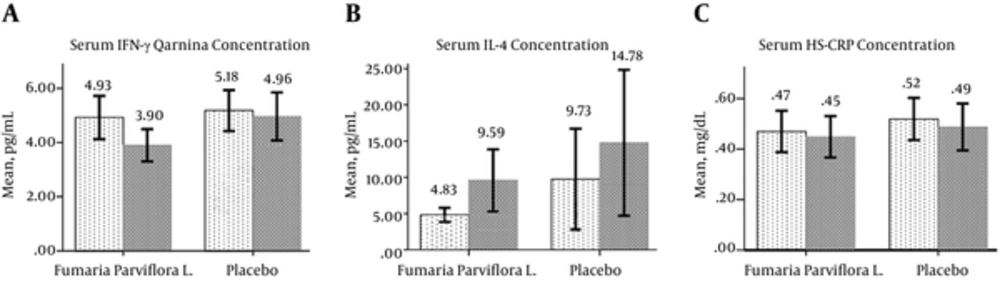
Before drug administration, IFN-γ was 4.93 ± 2.21 pg/ml in the FP group, which significantly decreased to 3.90 ± 1.65 pg/mL (P < 0.001). However, the decrease in the placebo group was not significant, changing from 5.18 ± 2.02 pg/mL to 4.96 ± 2.36 pg/mL (P = 0.604). The difference in IFN-γ between the two groups after the therapy period was significant (P = 0.04). Serum IL-4 levels were significantly elevated in the FP group, changing from 4.83 ± 2.72 pg/mL to 9.59 ± 11.82 pg/mL (P = 0.028). Although the serum IL-4 levels increased in the placebo group, this change was not significant (P = 0.100). The mean reduction of HS-CRP was not significant in the FP group (from 0.47 ± 0.227 mg/dL to 0.45 ± 0.227 mg/dL, P = 0.302) or in the placebo group (from 0.52 ± 0.239 mg/dL to 0.49 ± 0.250 mg/dL, P = 0.170) (Figure 3). No significant changes were observed between the pre- and post-treatment serum ALT levels in the FP group (P = 0.719) or in the placebo group (p = 0.918) nor were any significant changes in AST observed in the FP group (P = 0.720) and the placebo group (P = 0.154).
Light columns show pre-treatment measurements, and dark columns show post-treatment measurements. a: The serum IFN-γ concentration significantly decreased in the Fumaria parviflora L group (P < 0.001); b: The serum IL-4 concentration significantly increased in the Fumaria parviflora L group (P = 0.028); c: The serum HS-CRP concentrations before and after the study period were not significantly different in the Fumaria parviflora L group (P = 0.302) nor the placebo group (P = 0.170).
4.4. Adverse Events
In the FP group, four patients experienced gastric pain that led to two patients dropping out of the study. One patient complained of small rashes on both legs and feet, but this did not lead to drug discontinuation. In the placebo group, abdominal cramps in one patient and constipation in another patient led to two patients dropping out of the study. One patient in the FP group and six patients in the placebo group discontinued the drug because of severe pruritus that did not show any sign of easing. In this study, no significant differences were detected in pre- and posttreatment liver enzymes (ALT and AST).
5. Discussion
Our findings revealed that FP decreases pruritus in HD patients. There is a long history of FP use for pruritus and considerable use of herbal remedies by HD patients (31). However, to the best of our knowledge, this clinical trial is the first study to investigate the effects of FP on the relief of UP in ESRD patients undergoing HD. The results of this study demonstrated that repeated oral intake of FP during the treatment period is significantly more effective than a placebo for the reduction of UP despite the fact that the maximum effect of FP occurred after week 4.
At present, the immunohypothesis explaining the occurrence of UP is supported by several studies that point to generalized inflammation as the cause of UP (14). Kimmel et al. (32) found that CRP and IL-6 were higher in HD patients with UP compared with HD patients without UP. Kimmel et al. realized that, among the same patients, the ratio of Th1 (measured using CXCR3 as a chemokine receptor and IFN-γ) to Th2 (measured by CCR4 as a chemokine receptor and IL-4) were also higher. In addition, a higher serum level of ferritin and CRP as an acute phase protein and a lower serum level of transferrin and albumin as negative acute phase protein in HD patients with UP compared with the same patients without UP supported the probable role of inflammation in UP (33, 34). Additional evidence regarding this immunohypothesis is the decrease in UP following ultraviolet B (UVB) irradiation to even half of the body (35). This result may be due to the inhibition effect of UVB on Th1-mediated immune reactions (36).
FP has been used for the treatment of itching and some kidney diseases in traditional Persian medicine. A chemical analysis of FP has indicated the presence of FAEs with anti-inflammatory properties. MMF, the most effective component of FAEs, can upregulate IL-4 and IL-5 secretion by Th2 cells. Th2 cells prevent the development of inflammatory responses by downregulating the activities of Th1 cells, which results in reduction of IFN-γ (13). Another study showed that MMF affects the polarization of monocyte-derived dendritic cells that regulate the development of different Th subsets. This effect leads to the down regulation of Th1 responses and the lowered production of IFN-γ (29); as a result, MMF may decrease the level of IFN-γ and lead to anti-inflammatory responses through either of these two mechanisms.
Our study proved that IFN-γ level decreased significantly in HD patients who received FP compared with those who received placebo. Additionally, IL-4 increased significantly in the patients who received FP. There was, undoubtedly, reduction in the level of HS-CRP in the FP group (though this reduction was not significant). Interestingly, the severity of pruritus decreased significantly in the FP group, too. These results demonstrate that downregulation of Th1 cell differentiation, which is determined by decreased IFN-γ, or the up regulation of Th2 cell differentiation, which is determined by increased IL-4, might be the mechanism of the antipruritus effect of FP.
5.1. Limitations
There are some limitations that should be taken into account for the interpretation and generalization of this study’s results. The small sample size and short duration of the study are two main limitations of our study. Still, we planned to assess the patients for eight weeks weekly visits were maintained for interim evaluation. In addition, patients were followed for four weeks subsequent to exiting the study protocol. Another limitation was the use of a placebo for the control arm of the study. The next step should be to compare the efficacy of FP with conventional medications for UP.
5.2. Conclusion
Our report from this study shows that FP is safe and could decrease the severity of UP in HD patients. FP may be interpreted as a supporter of the immunohypothesis explanation for UP. We suggest that future studies should be conducted in a larger multicenter population for the evaluation of the effects of FP on UP and its likely mechanism.