1. Background
Plants or plant products have been used as a traditional medicine against many diseases by numerous civilizations (1). Primula, which is a medicinal plant bearing flowers, belongs to the family of Primulaceae and consists of 400 to 500 species. This species is found throughout the temperate Europe and Asia. Some of them are popular garden plants because of their colourful blossoms (1, 2). It is known that Primula herb has been used in folk medicine due to its antispasmodic, vermifuge, emetic, and astringent effects. Some of the Primula species are used traditionally to treat bronchitis, epilepsy, convulsions, cramps, spasms, paralysis, and rheumatic pains (1-4). Phenolic glycosides and saponins are the main compounds for the genus Primula (2). Many studies have shown cytotoxic, antibacterial, antiviral, antioxidant, antiangiogenic, anti-inflammatory and antimitotic effects of different species of Primula, and found that their biological effects are attributed to their phenolic contents (2, 4-8).
Oxidative stress is the corruption case of the balance between oxygen formation and antioxidant defense in the direction of the oxidants, and leads to cellular damage. DNA is an important target of oxidative attack, and reactive oxygen species (ROS) induced DNA damage has a close relationship with many pathological tables, such as cancer, heart diseases, and diabetes. Plants involve many antioxidant components such as polyphenolic compounds, and these compounds protect cells against the deleterious effects of ROS. The antioxidant effect of phenolic compounds is explained by their ability to donate electrons to ROS, chelating metal ions and stimulating antioxidant enzymes. Therefore, in the recent years, the exploration of new natural antioxidants has become a popular research area worldwide, and it seems that traditional medicine is a starting point for new discoveries (4, 9, 10). The antioxidant activity of Primula vulgaris extracts has been demonstrated by some reports previously (4, 11). However, to the best of our knowledge, no study has been conducted on its phenolics composition and antigenotoxic effects.
2. Objectives
The aim of this study was to determine the phenolics composition and the antioxidant activity of water extract of Primula vulgaris and to examine its probable preventive effects against H2O2-induced DNA damage in foreskin fibroblast cells for the first time.
3. Methods
3.1. Reagents
All phenolic standards, folin reagent, methanol, ethanol, sodium carbonate, 2,4,6-tri (2-pyridyl)-s-triazine (TPTZ), iron (III) chloride, potassium acetate, trolox, 2,2-diphenyl-2-picrylhydrazyl hydrate (DPPH), agarose, sodium chloride, sodium hydroxide, H2O2, trypan blue and phosphate buffer saline (PBS) tablets were purchased from Sigma (St. Louis, MO, USA). Acetonitrile, acetic acid, hydrogen chloride and dimethyl sulfoxide (DMSO) were purchased from Merck (Darmstadt, Germany). Penicillin-streptomycin and trypsin were obtained from Biological Industries (Kibbutz Beit Haemek, Israel). Eagle’s minimum essential medium (EMEM) was bought from Lonza (Verviers, Belgium), and fetal bovine serum (FBS) from Biochrom (Berlin, Germany).
3.2. Plant Collection and Extraction
Samples of Primula vulgaris were harvested in the summer in Trabzon in Eastern Anatolia region in Turkey. These flowers were air-dried at room temperature for 20 days and were powdered using blender and milling. The powder of the flowers were stored as packed in freezer bags at -20°C until tested. To prepare stock WEP, 1 g of the flower powder was weighed and mixed with 20 mL distilled water, and then the mixture was continuously stirred with a shaker at room temperature for 24 hours. Suspension was removed by centrifuging at 10,000 g for 15 minutes. Then, the supernatant was concentrated at 40°C in rotary evaporator (IKA-Werke RV05 Basic, Staufen, Germany) under reduced pressure. The dry residue was resolved with distilled water and filtered with 0.2 µm filter, and stored in 4ºC until used for further experiments.
3.2.1. Estimation of Total Phenolic Content (TPC)
The contents of the total phenolics of WEP were established by the spectrophotometric method (12) using gallic acid as a reference. The quantity of polyphenolic compounds was indicated as mg of gallic acid equivalents (GAE)/g sample.
3.2.2. Estimation of Reducing Power
The reducing power of WEP was established by FRAP assay (13) using trolox as a reference, and the results of FRAP analysis were indicated as µmol trolox equivalents (TE)/g sample.
3.2.3. Free Radical Scavenging Activity
The free radical scavenging activity of WEP was established by DPPH assay (14) using trolox as a positive control. The percent reduction of the DPPH radical was calculated using the following equation:
DPPH inhibition (%) = 100 - (Asample/Acontrol) × 100
The SC50 value (the concentration of the compound required the reduction of the absorbance of DPPH by 50%) was estimated graphically in five different concentrations. SC50 value of the extract was stated as mg/mL (15).
3.3. HPLC Analysis of Phenolic Compounds
Thirteen standards were used for HPLC analysis as follows: Gallic acid, protocatechuic acid, ρ-OH benzoic acid, vanillic acid, caffeic acid, ρ-coumaric acid, syringic acid, ferulic acid, trans-cinnamic acid, catechin, epicatechin, rutin, and luteolin. The propylparaben was used as an internal standard (16).
HPLC analysis of phenolic compounds was performed using a reverse phase column (150 × 4 mm i.d, 5 µm) (Fortis, Cheshire, UK) on a gradient program with two solvents system [A: 2% acetic acid in water; B: 0.5% acetic acid in acetonitrile:water (1:1)] at a constant solvent flow rate of 1.2 mL/min on a HPLC system (Thermo Finnigan Surveyor, USA) (17). Injection volume was 25 µL. Signals were detected at 232, 246, 260, 272, 280, 290, 308 and 328 by diode array detector (DAD) and at 280 nm by UV detection. Column temperature was maintained at room temperature, 25°C. Identification of compounds was performed comparing retention times and spectral data with those of pure standards. Calibration curves of the standarts were used for quantitation. Data are stated as mean ± SD for three replicates.
3.4. Cell Culture
Human foreskin fibroblast cells were purchased from American Type Culture Collection (Manassas, VA, USA). Cells were grown in EMEM supplemented with 10% FBS, 2 mM glutamine, 1% penicillin and streptomycin in disposable plastic flasks, at 37°C. All experiments were carried out using foreskin fibroblast cells between the first and sixth culture passages.
3.4.1. Determining H<sub>2</sub>O<sub>2</sub> Concentration
A total of 2 × 105 fibroblast cells were cultured in a T-25 flask. After 24 hours, fibroblasts were handled with 10 - 30 μM H2O2 for five minutes to determine the concentration resulting in DNA damage, but not toxicity. After incubation, cells were trypsinized and centrifuged for comet assay.
3.4.2. Determining WEP Concentration
Fibroblasts were pre-incubated with various concentrations of WEP (100, 250, and 500 µg/mL) for 60 minutes. After that, flasks were washed with PBS, and cells were handled with 20 µM H2O2 for five minutes. Following incubation, flasks were washed, trypsinized, and centrifuged for comet protocol.
3.4.3. Cell Viability
Trypan blue dye exclusion test was used to evaluate cell viability (18). After treatments and trypsinizations, the cell suspensions were mixed with trypan blue solution, and cell viabilities were determined by direct counting of the cells in a neubauer chamber under an inverted microscobe (Nikon Eclipse TS100, Tokyo, Japan). One hundred of cells were counted per group and the experiment was run in triplicate.
3.4.4. Comet Assay
The alkaline version of comet assay was used to determine the DNA damage (19) with slight modifications. A total of 75 µL of cell suspension was mixed with 75 µL of 1% low melting agarose, and was rapidly spread on the slides precoated with normal melting agarose (0.75%). The slides were covered with coverslips, and agarose layer was allowed to solidify at 4°C for five minutes. After removal of the coverslips, slides were immersed into cold, freshly made lysing solution (2.5 M NaCl, 100 mM EDTA, 10 mM Tris, and 1% (v/v) Triton X-100) for one hour at 4°C. Then the slides were removed from the lysing solution and placed in a horizontal gel electrophoresis tank filled with fresh electrophoresis buffer (300 mM NaOH, 10 mM Na2EDTA and 1% (v/v) DMSO (pH 13.1). The slides were left for 30 minutes at 4°C to allow unwinding of DNA. Electrophoresis was then carried out at room temperature in the same electrophoresis buffer for 25 minutes at 1 V/cm and 300 mA. After electrophoresis, the slides were incubated in fresh neutralization buffer for 15 minutes (0.4 M Tris, pH 7.5), before staining with ethidium bromide (20 µg/mL). For each treatment condition, 100 randomly selected cells from each slide were evaluated for DNA damage visually using a 40 × objective on a fluorescent microscope (Nikon Eclipse E800, Tokyo, Japan). The selected cells were classified between 0 and 3, from non-damaged to most damaged, according to tail length. Excessively long tails and DNA spectra scored 4 were not included. All slides were scored with the following formula (18, 20) with a maximum damage possibility of 300:
Comet score = (1 × n1) + (2 × n2) + (3 × n3) (n: cell number for every score)
The percent reduction of DNA damage by WEP was determined using the following equation (18) (Equation 1):

Where A: Comet score of cells treatment with only H2O2 (positive control), B: Comet score of cells with antigenotoxic treatment (WEP + H2O2) and C: Comet score of the control cells (no treatment).
3.5. Statistical Analysis
All experiments were carried out triplicate. The results were given as mean ± standard deviation. Compatibility with normal distribution was determined, using the Kolmogorov-Smirnov test. One-way ANOVA was used to compare the differences among the groups. Statistical significance was set at P < 0.05.
4. Results
4.1. Antioxidant Properties of WEP
TPC, FRAP, and DPPH results of WEP were summarised in Table 1.
4.2. HPLC Profile of Primula vulgaris
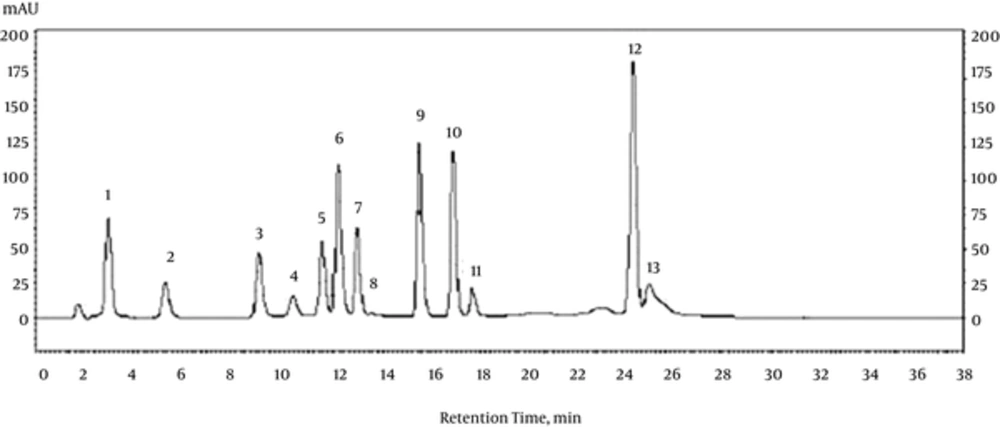
The chromatogram of phenolic standards are presented in Figure 1. The phenolic compounds found in Primula vulgaris are summarised in Table 2, and the values are stated in µg/g sample. ρ-coumaric acid and rutin were most abundant compounds in Primula vulgaris (Table 2).
| Phenolic Compound Assignment | Retention Time, min | Peak Area, % | Amount, µg/g FW |
|---|---|---|---|
| Gallic acid | 3.12 | 0.22 | 3.23 ± 0.06 |
| Protocatechuic acid | 5.42 | 2.09 | 30.71 ± 0.03 |
| ρ-OH benzoic acid | 9.21 | 5.21 | 76.56 ± 0.17 |
| Catechin | 10.74 | 3.68 | 53.97 ± 1.31 |
| Vanillic acid | 11.82 | 4.43 | 65.01 ± 0.10 |
| Caffeic acid | 12.52 | 3.63 | 53.19 ± 0.27 |
| ρ-coumaric acid | 13.22 | 57.8 | 848.03 ± 2.70 |
| Rutin | 13.65 | 22.94 | 336.52 ± 15.32 |
| Syringic acid | 15.86 | - | ND |
| Epicatechin | 17.12 | - | ND |
| Ferulic acid | 17.88 | - | ND |
| Trans-cinnamic acid | 24.58 | - | ND |
| Luteolin | 25.21 | - | ND |
Phenolic Composition of the Water Extract of Primula vulgarisa
4.3. Cell Viability and Comet Analysis
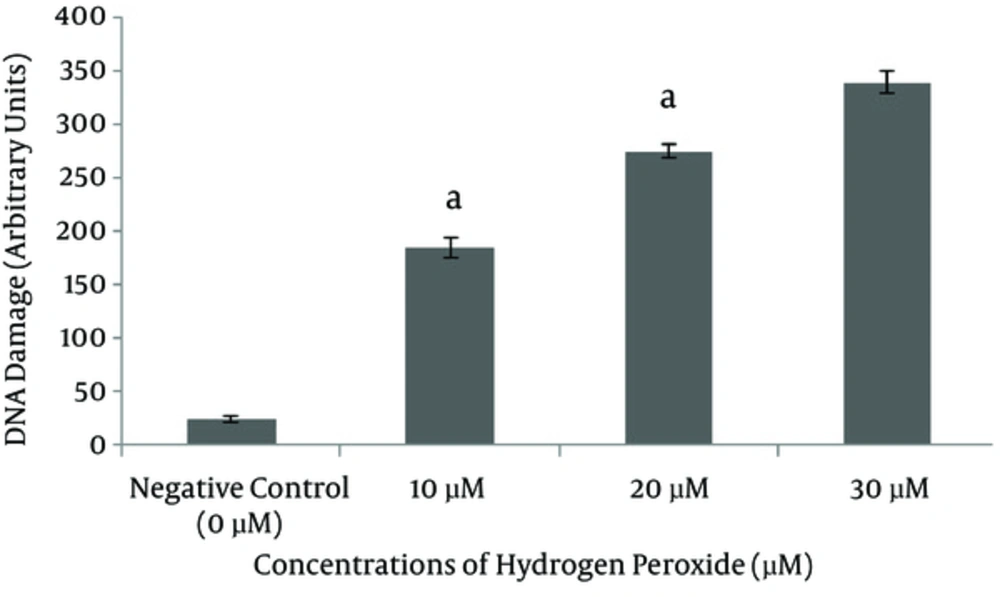
Cell viabilities were found to be over 98% in all groups. A significant increase was induced in DNA damage with increasing the concentration of H2O2 (10, and 20 µM; P < 0.001 for all; Figure 2); H2O2 of 20 μM, which produces approximately a 300 comet score in five minutes, was used as the damage concentration in the following assay.
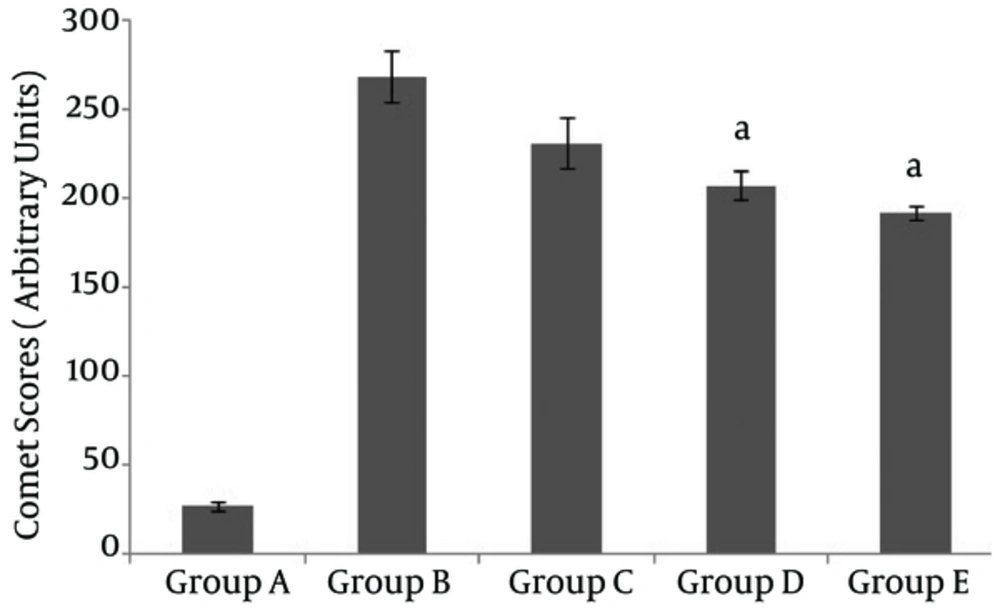
Cells were pretreated with WEP, and comet scores were compared after damage with 20 μM H2O2. Only the concentrations of 250 and 500 µg/mL WEP decreased DNA damage significantly (P < 0.001) (Figure 3). The percentage reductions of H2O2-induced DNA damage by WEP were 16%, 25%, and 32% for concentrations of 100, 250, and 500 µg/mL WEP, respectively.
5. Discussion
Oxidative stress is caused by ROS and is the main by-product formed in the cells of aerobic organisms and has a close relationship with many pathological tables, such as cancer, heart diseases, and diabetes. Therefore, antioxidant activity is an essential feature for human health. It is believed that many of the biological functions may originate from this feature. Phenolics in natural products might protect the humankind against chronic diseases with their antioxidant action. Determining the antioxidant activity of the tested natural product is therefore accepted as a starting point for more comprehensive studies (21). Many in vitro assay are used to determine the antioxidant capacity of herbal extracts, and at least two different methods are recommended (22, 23). Total phenolics content is often used to examine the antioxidant properties of plant extracts since it is useful, rapid, and cost-effective (24). A direct relationship was found between the total polyphenolic contents and antioxidant capacity of many fruits and vegetables (25). The FRAP method is frequently preferred to estimate the antioxidant power of a compound (26). The DPPH scavenging assay is one of the most used methods because it reacts directly and rapidly to antioxidants in a simple manner (23). Consequently, we preferred to determine the antioxidant capacity of WEP with these three different assays. TPC, FRAP, and DPPH results of WEP are presented in Table 1. Orhan et al. demonstrated that the TPC value of water extract of Primula vulgaris is 7.55 mg GAE/g extract (4). Also, Demir et al. demonstrated that the TPC value of water extract of Primula vulgaris is 89.6 μg GAE/mg extract. Besides, the FRAP and DPPH inhibition values of water extract of Primula vulgaris are 43% and 99.5% for the concentration of 45 μg/mL in the same study, respectively (11). The difference between our antioxidant activity results and those of other studies may be due to the plant species, type of extraction method, geographic region, harvest season, and post-harvesting conditions.
Several reports have described the use of HPLC with DAD for characterization and quantification of phenolic composition. It is known that HPLC presents higher robustness, reproducibility, and sensitivity, and it interfaces easily with a great range of detectors (27-29). Therefore, HPLC-DAD system was preferred for phytochemical analysis in this study, and eight phenolic compounds (gallic acid, protocatechuic acid, ρ-OH benzoic acid, catechin, vanillic acid, caffeic acid, ρ-coumaric acid and rutin) were determined in the WEP (Table 2). The results of previous researches revealed that the genus Primula is rich in polyphenolic compounds, such as quercetin, kaempferol, isorhamnetin, rutin, methoxy derivatives of flavone, and gallic acid (10, 30-32). Phenolic compounds are secondary metabolites of plants and can exhibit many medical properties (antioxidant, cardioprotective, anti-inflammatory, antimicrobial, anticancer, anti-ageing, etc.). Thus, polyhenols or natural products including polyphenols have allured increasing interest as potential agents for preventing and treating many oxidative stress-related diseases, such as cardiovascular diseases, cancer, ageing, diabetes mellitus, and neurodegenerative diseases (33, 34). Primula vulgaris is a medicinal plant, which has traditionally been used to treat many diseases (1-4). The positive therapeutical properties of Primula vulgaris in traditional medicine may arise from these phenolic compounds. There was not any overlap between our phenolic composition results and the literature data. This situation may arise from the plant species and the number and kind of the used standards. We believe that the phenolic composition of P. vulgaris might offer higher standard compounds should be changed with might reveal with further standard compounds.
The comet assay is frequently used to detect DNA damage in cellular level due to its cheapness, easiness, and more sensitivity than the other assays (18, 19). Moreover, it has been considered as a favorable assay to evaluate the capacity of phytochemicals to protect cells against genotoxic agents (35, 36). Therefore, we preferred to determine DNA damage using comet assay in this study. Methyl methanesulfonate, H2O2, ferrous sulfate, tert-butyl hydroperoxide, and doxorubicine have been reported to cause in vitro DNA damage in cells in antigenotoxicity studies (18, 20, 37, 38). In this study, H2O2 was used to generate oxidative DNA damage in fibroblast cells. H2O2 is a hydrofobic molecule; It can therefore diffuse into the cytoplasm quite easily and can be rapidly transformed into hydroxyl radicals by the Haber-Weiss or Fenton reactions. Hydroxyl radical is the most detrimental type of ROS and can induce various types of DNA damage, such as strand break, alkali-labile site, oxidized purine and pyrimidine (18). In this study, comet scoring was performed using a scale of 0 (no damage) to 3 (most damage) in visual analysis in the comet assay between 10 and 30 μM H2O2 damaging concentrations. A significant increase was induced in DNA damage with increasing the concentration of H2O2 (10, and 20 µM; P < 0.001 for all; Figure 2). Maximum comet score is 300 on this scale, and the H2O2 concentration was therefore selected as 20 μM. Excessively long tails and DNA spectra scored at 4 were not included in this study, so 30 μM H2O2 was not preferred as an optimum concentration of H2O2. Previous reports have demonstrated that the damaging concentration of H2O2 in fibroblast cells changes between 10 and 100 μM. Thus, we used both concentrations of H2O2 (18, 39, 40) and incubation time of H2O2 (5 Min), which were compatible with previous literature (18, 41, 42). In this study, Primula vulgaris extract, rich in phenolic compounds, was investigated for its protective effect on H2O2-induced oxidative DNA damage in foreskin fibroblast cells. The pretreatment time (1 hour) of fibroblast cells with WEP was established according to similar studies conducted on the antigenotoxic effect of natural products (18, 41, 43). Cells were pretreated with different concentrations of WEP before H22O2 treatment, and comet scores were compared with only 20 μM H2O2 treatment group (the positive control). The only concentrations of 250 and 500 µg/mL WEP decreased DNA damage significantly (P < 0.001) (Figure 3). The percentage reductions of H2O2-induced DNA damage by WEP were 16%, 25%, and 32% for concentrations of 100, 250, and 500 µg/mL WEP, respectively. Due to the pro-oxidant effect of the extract at greater than 500 µg/mL concentration on fibroblast cells, 500 µg/mL concentration was selected as the highest concentration following the preliminary tests. In our study, WEP did not return H2O2-induced DNA damage to negative control levels. This, in part, may be due to the pre-incubation time of one hour being too short. To date, no study has reported the effect of Primula vulgaris extract on oxidative DNA damage by the comet assay. However, only Aslam et al. demonstrated that the ethanolic leaf extract of Primula denticulata prevents DNA damage against oxidative stress on calf thymus DNA (10). We believe that at this time the antigenotoxic effect of P. vulgaris should be investigated both in vitro condition with different treatment types (post or simultaneous) and in vivo condition.
Many plants contain antioxidant components such as polyphenolic compounds, which protect cells against the detrimental effects of ROS (9). The antioxidant property of polyphenolic compounds is attributed to their ability to donate electrons to ROS (44). Aherne and O’Brien reported that preincubation with three flavonoids (quercetin, myricetin, and rutin) significantly protect human colon and liver cells against H2O2-induced DNA damage without affecting cell viability and activity of antioxidant enzymes (catalase and superoxide dismutase) (45). Also, (+)-catechin exhibits a protective effect against heterocyclic amines-induced oxidative DNA damage in human liver cells and it is more efficient than (-)-epicatechin in preventing DNA damage (36). In addition, the protective effects of benzoic acid, caffeic acid, coumaric acid, gallic acid and vanillic acid against DNA damage have been demonstrated in several in vitro and in vivo studies (45-48). In this study, many of these compounds were determined in the extract, and the antigenotoxic effects of WEP may arise from its phenolic content.
To our knowledge, this was the first study on phenolics composition, antioxidant and antigenotoxic effect of Primula vulgaris extract. Further studies are required for the isolation and identification of individual phenolic compounds in the extracts. Moreover, the phytochemical studies together with biological activity investigations are essential to reach a complete understanding of the medicinal applications.