1. Background
The widespread problem of fibrosis develops chronic injury, viral hepatitis, fatty liver, alcoholism, metabolic and genetic disorders (1, 2). Among different resident cells within the liver, human hepatic stellate cells (HSCs) play a key role in the development of liver fibrosis (2). Under normal circumstances, HSCs are in a quiescent state and play a role in retinoid storage. However, following chronic damage they become activated and transverse into a myofibroblast phenotype (3-5). In the activated form, HSCs act as the main source of pro-inflammatory cytokines (e.g., TGF-β) and fibrogenic factors such as different types of collagen, matrix metalloproteinases (MMP) and tissue inhibitor of metalloproteinase (TIMP) (6-9). TGF-β, the most profibrogenic cytokine in the liver, shows paracrine/autocrine effects on HSCs. Besides its function in activation of HSCs, TGF-β also stimulates the production of various matrix components including collagen type I (COLI), which is the major fibrous protein in the extracellular matrix. Establishment of activated HSCs leads to the accumulation of extracellular matrix, malfunction in tissue remodeling and ultimate fibrosis development (3, 10).
Despite significant advances in understanding the molecular mechanisms of fibrosis, only a limited number of antifibrotic drugs are available (2, 10). Among different molecular strategies for the fibrosis therapy, specific targeting of activated HSCs has been addressed by numerous scientists (11). Ideally, HSC targeted antifibrotic agents that aim to block or reverse their activation seem to be rational goals for the future fibrosis therapy.
Based on the chronic nature of fibrosis, long-term therapy with safe natural components seems more applicable than detrimental chemical components (2, 12). A number of natural agents such as silymarin (13, 14) and 18α-glycyrrhizin (15) have been shown to be effective on alleviation of liver fibrosis by controlling HSC functions. Silymarin, an extract from the milk thistle plant (Silybum marianum) has been used as general remedy for the liver disease since ancient times (16-19). This compound consists of at least seven flavonolignan complexes. Among these, silybin A has a special importance for healing the liver (20). Recently, both silymarin and silybin A have been employed in clinical experiments with the aim to ameliorate the process of liver fibrosis (21).
Another useful herbal medicine, 18α-glycyrrhizin is a major pentacyclic triterpenoid glycoside of the licorice root (22-24) Although this medicine has been widely used for the treatment of chronic hepatitis C infection in different trials, its detailed impacts on liver fibrosis and HSCs remain elusive (15)
2. Objectives
While the healing effects of the aforementioned components on liver damage have been evaluated, their antifibrotic roles were not compared together in vitro in a characterized human HSC line (LX-2). Furthermore, there are a limited number of studies that have compared their antiproliferative impacts on activated HSCs. An evaluation of the therapeutic and prophylactic manners of these natural components is another point of view considered in this study. Therefore, the current study aimed to compare the antiproliferative and antifibrogenic effects of three herbal medicines (silymarin, silybilin A and 18α-glycyrrhizin) on TGF-β-activated LX-2 cells under prophylactic and therapeutic conditions.
3. Methods
3.1. Cell Culture
The LX-2, an immortalized human HSC line, was kindly gifted by Professor Scott Friedman, Mount Sinai School of Medicine, New York. This cell line exhibits typical features of partially active stellate cells by expression of desmin and glial acidic fibrillary protein (GAFP) beside responsiveness to platelet-derived growth factor (PDGF) and TGF-β1, thus can be regarded as a key target cell for the liver fibrosis therapy (2). The cells were cultured and maintained in 25 cm2 tissue culture flasks (Nunclone, Denmark) in a low glucose Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco, CA) supplemented with 5% fetal bovine serum (FBS, Sigma, St. Louis, MO), 100 U/mL penicillin-streptomycin (Gibco) and 2mM L-glutamine at 37°C in 5% CO2 air humidified atmosphere. During cell activation by TGF-β1, the amount of FBS in media was adjusted to less than 2%. After passage 25, the LX-2 cells were usually discarded and they were always checked for TGF-β1 secretion and COLI gene expression following leptin stimulation (9).
3.2. Component Preparation and Cell Viability
Silymarin, silybin A and 18α-glycyrrhizin (Sigma) were dissolved in dimethyl sulfoxide, (DMSO, Sigma). The cytotoxic effect of different concentrations of these components (0.1, 1, 10, 25, 50 and 100 μg/mL) on quiescent LX-2 cells was evaluated by MTT [3-(4, 5-dimethylthiazole-2-yl)-2, 5-diphenyl tetrazolium bromide] colorimetric assay as previously described (25). In brief, 7.5 × 103 cells in 100 μL were seeded into 96-well culture plates and then cells were treated with the components at different concentrations in triplicate. A series of triplicate wells containing DMSO, at the concentrations equal to test wells was considered as the negative control. Cells were incubated at 37°C in humidified CO2 incubator for 48 hours. The supernatants of cells were then replaced with a mixture of 90 µL fresh medium and 10 µL MTT solution (Sigma) at 5 mg/mL and the cells were incubated for 3 hours at the same condition. Afterward, 100 µL DMSO was added to each well. The plate was put on horizontal rotator and dark condition to increase dissolving of formazan crystals. The optical density (OD) of each well was then determined at dual wavelengths of 570 and 630 nm on a microplate reader (BioTekElx, Winooski, VT). Viability percentage was calculated as follows; % of viable cells = (OD of treated sample/OD of negative control) × 100.
3.3. Cell Proliferation Assay on Activated LX-2 Cells
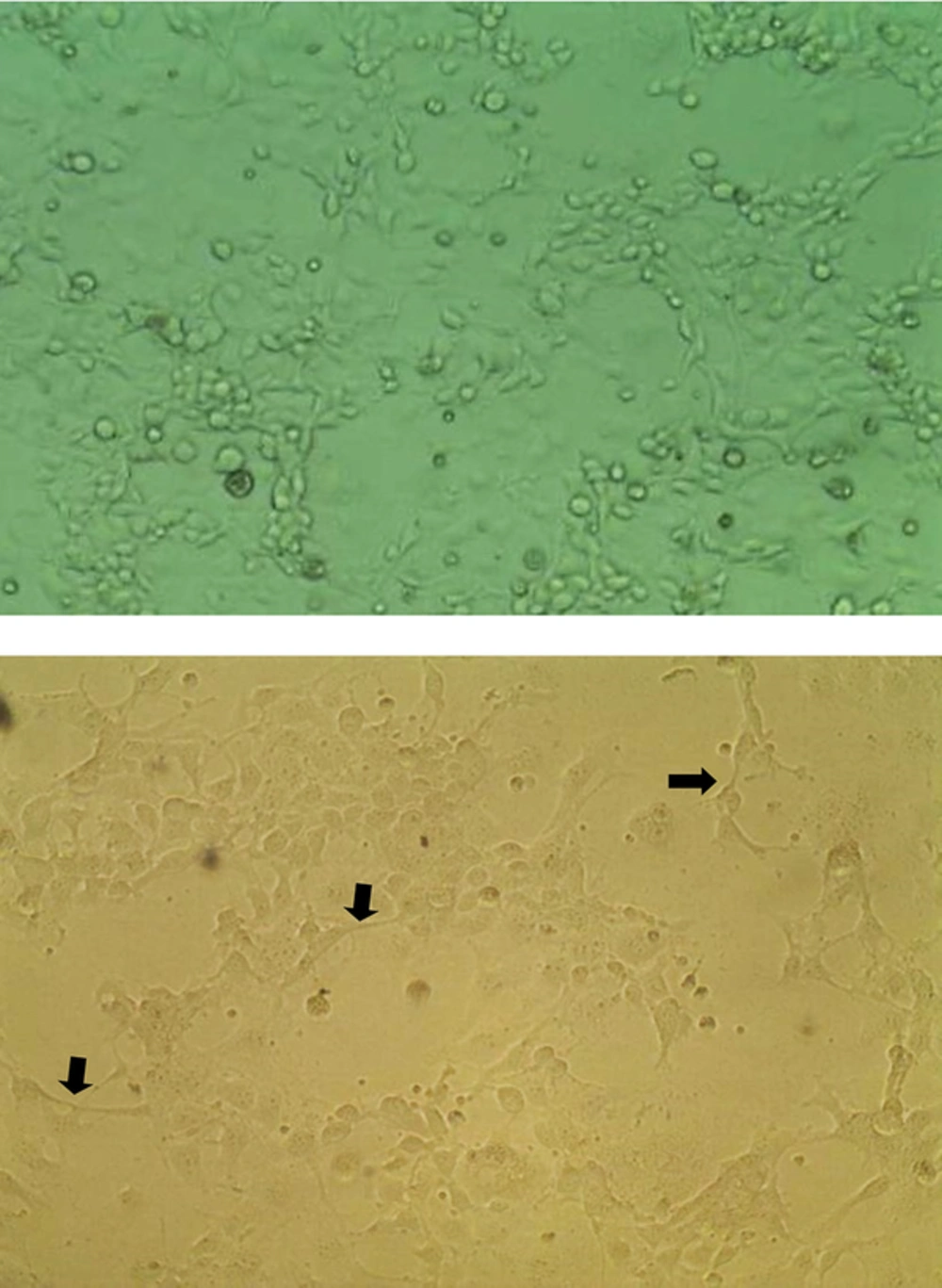
After finding the highest noncytotoxic concentrations of the components on quiescent LX-2 cells, the cells were activated with TGF-β1. The activated phenotype happened as cells harboring surface projections appeared on culture plate (Figure 1). The effects of 25 and 50 μg/mL of components on cell growth of activated cells was examined by the MTT assay as mentioned above in two sets of experiments; a prophylactic experiment in which the components were added 6 h before TGF-β1-cell activation, and a therapeutic experiment in which the components were added 12 hours after TGF-β1-cell activation.
Cells containing DMSO at the highest concentration used in the tests (e.g., 0.05%) was considered as a positive control when they were treated only with TGF-β1 (TGF-β1-alone treated cells) and as a negative control when no treatment was performed (untreated). The percentage proliferation was calculated as follows; % proliferation = (OD of treated sample/OD of negative control) × 100. The absorbance of solubilized formazan in TGF-β1-alone treated cells was taken as 100% proliferation. Experiment was carried out in triplicate and repeated at least three times.
Of note, in order to activate LX-2 cells, cells were first serum starved and supplemented with 0.2% FBS for 48 h and then a home-made TGF-β1 in concentration of 5 ng/mL was added to the cultures. For preparation of home-made TGF-β1, in another setting, LX-2 cells were seeded into 75 cm2 culture flasks in DMEM and then when cells reached more than 80% confluency, they were supplemented with 75 ng/mL leptin profibrogenic hormone (Sigma). After 48 hours incubation at 37°C in humidified CO2 incubator, the supernatant that contained TGF-β1 was collected and passed through 5-kDa-cutoff Amicon ultrafiltration column (Milipore Billerica, MA). The concentration of prepared TGF-β1 was determined by enzyme-linked immunosorbent assay (ELISA). The prepared TGF-β1 stored at - 80°C until use.
3.4. ELISA Assay for TGF-β1 Measurement
LX-2 cells were activated with TGF-β1 and treated with 25 μg/mL of components in two sets of prophylactic and therapeutic experiments, as mentioned for the MTT assay. Nearly 36 hours after LX-2 cell activation with TGF-β1, the cells were removed for RNA extraction and the culture supernatants were collected for measurement of the released TGF-β1 using a human-mouse TGF-β1 ELISA kit (eBioscience, CA) according to the manufacture’s instruction. All the samples were first acid-activated and before starting the method and background, the TGF-β1 level (due to adding home-made TGF-β1 for LX-2 activation) was omitted from the final analysis. The absorbance was measured at 450 nm by the ELISA reader (BioTek Elx) and then the cytokine level was calculated by interpolation from a standard curve.
3.5. RNA Extraction and Reverse Transcription
Total RNA was extracted from treated cells using the Trizol reagent (Invitrogen Inc. Carlsbad, CA) according to the manufacturer’s standard protocol. The extracted RNA was eluted in 50 µL of DEPC-water then stored at - 80°C until use. Before cDNA synthesis, total RNA was treated with RQ1 DNase (Progma, Madison, WI) to avoid residual DNA contamination. The cDNA synthesis was carried out by the RocketScript RT premix kit (Bioneer, South Korea) based on the submitted manual. Briefly, extracted RNA in addition to the mixture of random hexamer and oligo-dT was added into reaction tubes, then reaction was performed for 1 min at 60°C followed by 60 minutes at 45°C and a 5-minute enzyme inactivation step. The prepared cDNA stored at -20°C condition until use.
3.6. Real-Time Polymerase Chain Reaction for Gene Expression Analysis
The primer pairs for COLIA1, TIMP-1, MMP-2 and GAPDH were designed by the NCBI Primer-BLAST online software as listed in Table 1 (26). The human GAPDH as a reference gene for normalization, H2O as negative control of reactions and RNA extract as negative control of cDNA synthesis enrolled in all PCR runs to make sure of experiments accuracy. The mRNA levels were relatively quantified using SYBR green I master mix (Bioneer, Daejeon, Korea) by the help of the IQ5 real-time PCR system (Bio-Rad Laboratories, Hercules, CA). The reaction mixtures prepared according to recommended protocol and amplification reaction performed in a 40 cycles of 10 s at 94°C for denaturation and 25 seconds at 58°C for annealing and extension steps. After each PCR run, melting curve analysis and gel electrophoresis was carried out to confirm specific amplification of targets. Amplification signals for different samples were normalized to the respective GAPDH signals and then the delta-delta CT (2-ΔΔCT) method was used to compare mRNA levels of tests versus negative control (untreated) which finally represented as fold change.
| RNA | Primer Sequences | Size (bp) |
|---|---|---|
| GAPDH | 5’- GAA GGT GAA GGT CGG AGT C-3/ (sense) | 226 |
| 5’- GAA GAT GGT GAT GGG ATT TC-3/ (antisense) | ||
| TIMP-1 | 5’- TAC TTC CAC AGG TCC CAC AAC-3/ (sense) | 202 |
| 5’- GTT TGC AGG GGA TGG ATA AAC-3/ (antisense) | ||
| MMP-2 | 5’- ATG ACA TCA AGG GCA TTC AGG AG-3/ (sense) | 135 |
| 5’- TCT GAG CGA TGC CAT CAA ATA CA-3/ (antisense) | ||
| COLIA1 | 5’- GAG GGC CAA GAC GAA GAC ATC-3/ (sense) | 140 |
| 5’- CAG ATC ACG TCA TCG CAC AAC-3/ (antisense) |
3.7. Statistical Analysis
Statistical analysis was performed using the GraphPad Prism software (San Diego, CA). They were analyzed using one-way ANOVA to evaluate the difference between groups. Tukey’s post- hoc test was used to determine the statistical significance between controls and component-treated groups. P < 0.05 was considered as statistically significant.
4. Results
4.1. Effects of Silymarin, Silybin A and 18α-Glycyrrhizin on LX-2 Viability and Cell Proliferation
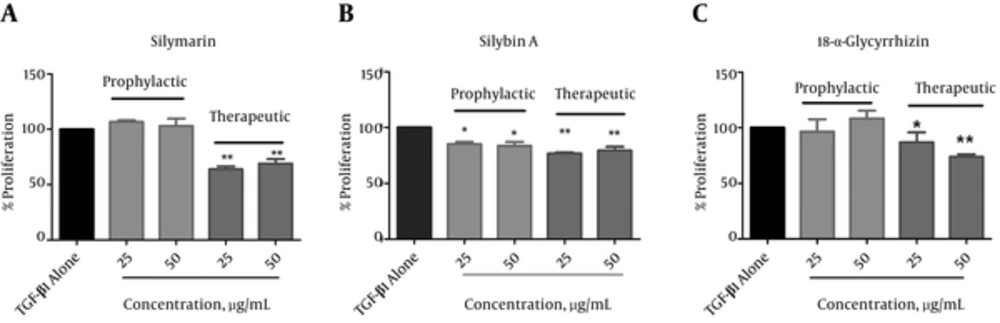
The cytotoxicity of these natural components on quiescent LX-2 cells was examined by the MTT assay. Silymarin, silybin A and 18α-glycyrrhizin at concentrations up to50 µg/mL did not show any cytotoxic effects (results not shown). Thus, we used two noncytotoxic concentrations of the components (25 and 50 µg/mL) for the next experiments. To determine the antiproliferative effects of silymarin, silybin A and 18α-glycyrrhizin on activated LX-2 cells, we incubated these cells with 25 and 50 µg/mL concentrations of the components prior to (prophylactic) and after (therapeutic) addition of TGF-β1.
The results showed that in the prophylactic experiment, silybin A significantly suppressed proliferation of LX-2 cells. The percentage of cell proliferation reduced to 85.1 ± 3.1% at the 25 µg/mL concentration and 83.5 ± 5.8% at the 50 µg/mL concentration of TGF-β alone-treated cells (P < 0.05; Figure 2B). Under this condition, silymarin and 18α-glycyrrhizin showed no significant effects on cell proliferation as depicted in Figure 2A and C, respectively. With respect to the therapeutic experiments, all components displayed significant antiproliferative effects on activated LX-2 cells (Figure 2). The percentage of cell growth inhibition at the 25 µg/mL concentration was 63.9 ± 4.7% (silymarin), 76.8 ± 1.6% (silybin A) and 86.8 ± 15.2% (18α-glycyrrhizin) of cells treated with only TGF-β (P < 0.05). The percentages of cell growth inhibition at the 50 µg/mL concentration were 69.3 ± 6.5% (silymarin), 79.4 ± 5.7% (silybin A) and 73.9 ± 3.8% (18α-glycyrrhizin, P < 0.01). There were similar results in this analysis for silymarin, silybin A and 18α-glycyrrhizin at 25 and 50 µg/mL concentrations. Therefore, we used the 25 µg/mL concentrations of silymarin, silybin A and 18α-glycyrrhizin for the next experiments.
Cells were treated with silymarin (A), silybin A (B) and 18α-glycyrrhizin (C) before (prophylactic) and after (therapeutic) activation by TGF-β and then cell proliferation was examined by the MTT assay. The proliferation of TGF-β1-alone treated cells (black bar) was taken as 100%. Each bar indicates mean ± standard deviation of % proliferation from at least three experiments. * P < 0.05 and ** P < 0.01 showed significant differences with TGF-β alone-treated cells.
4.2. Effects of Silymarin, Silybin A and 18α-Glycyrrhizin on TGF-β1 Production by Activated LX-2 Cells
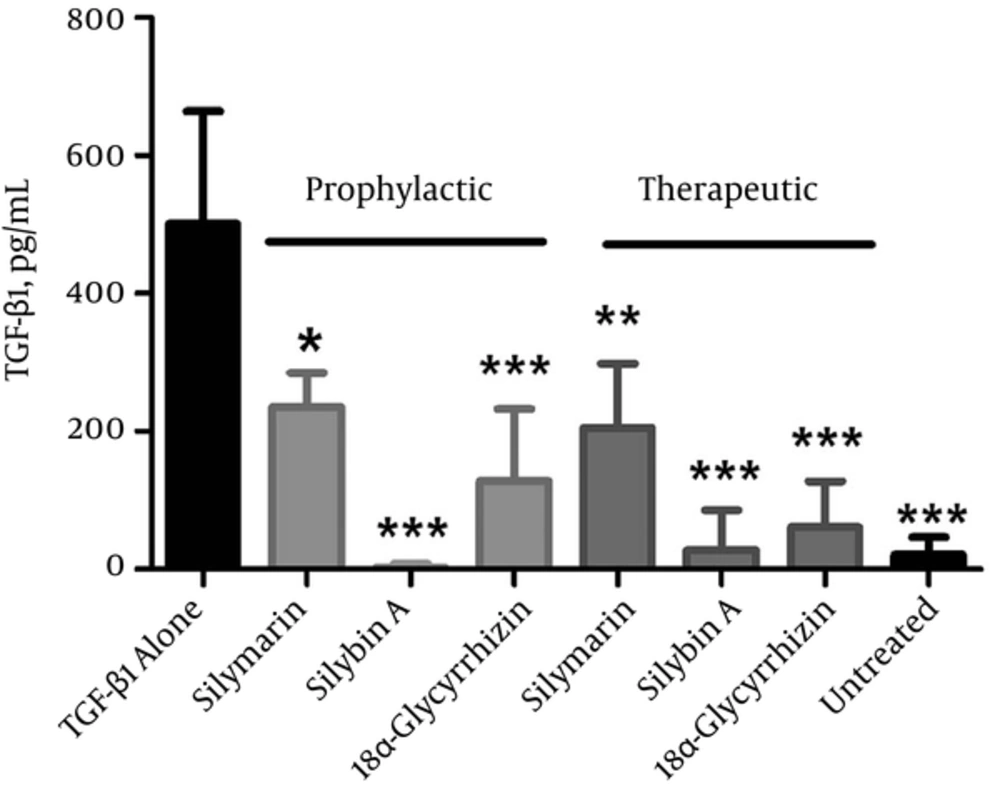
We evaluated theTGF-β1 cytokine production in the culture supernatants of cells treated under both prophylactic and therapeutic conditions according to ELISA (Figure 3). The results indicated that all three components significantly inhibited TGF-β1 production during prophylactic and therapeutic treatments. Under the prophylactic condition, the level of TGF-β1 reduced from 501 ± 163.4 pg/mL in TGF-β1-alone treated cells to 235.7 ± 49.4 pg/mL (silymarin; P < 0.05), 3 ± 5.2 pg/mL (silybin A; P < 0.001) and 128.3 ± 104 pg/mL (18α-glycyrrhizin; P < 0.001). Similarly, the therapeutic experiment showed a significant difference in the TGF-β1 production between component-treated cells and TGF-β1-alone treated cells. The level of TGF-β reduced to 205 ± 93.4 pg/mL (silymarin; P < 0.01), 28.5 ± 57 pg/mL (silybin A; P < 0.001), and 60.7 ± 67.1 pg/mL (18α-glycyrrhizin; P < 0.001). These results showed that silybin A under prophylactic and therapeutic conditions exhibited the most significant reduction in TGF-β1 levels (P < 0.001), whereas silymarin had the lowest inhibitory effect on TGF-β production in both conditions (P < 0.05).
Cells were treated with 25 µg/mL silymarin, silybin A and 18α-glycyrrhizin before (prophylactic) and after (therapeutic) activation by TGF-β and then cytokine measurement in the cell supernatant was performed by enzyme-linked immunosorbent assay. TGF-β1-alone treated cells and cells with no treatment (untreated) were used as controls. Each bar is representative of the mean ± standard deviation from at least three measurements and final results enrolled after subtraction of background TGF-β1 (had been added for cell activation). *P < 0.05, ** P < 0.01 and *** P < 0.001 showed a significant difference with TGF-β1 alone-treated cells.
4.3. Effects of Silymarin, Silybin A and 18α-Glycyrrhizin on the Expression of Profibrogenic Genes by Activated LX-2 Cells
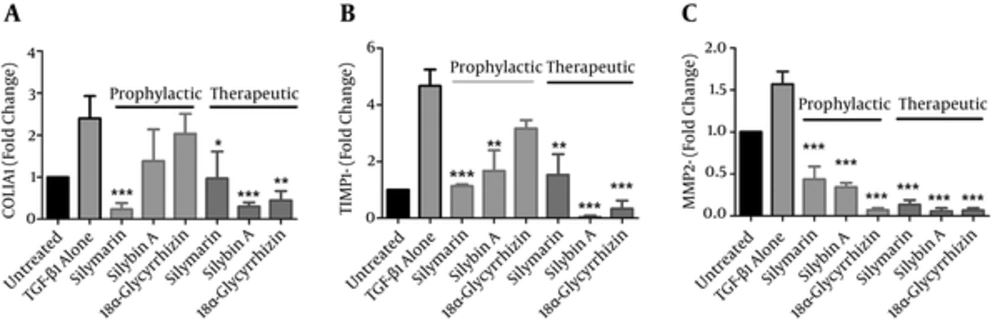
We sought to identify the influence of each component on the expression of fibrosis-related molecules by investigating the effects of these components on gene expressions of collagen type I-alpha-1 (COLIA1), TIMP-1 and MMP-2 under prophylactic and therapeutic conditions. mRNA expression levels for these genes were quantified by the real-time polymerase chain reaction (PCR) after exposure of LX-2 cells to 25 µg/mL each of silymarin, silybin A and 18α-glycyrrhizin. As shown in Figure 4, TGF-β-alone treated LX-2 cells displayed an increase in gene expression of fibrogenic molecules compared to the untreated cells. We determined that all three components under therapeutic conditions reversed LX-2 activation by decreasing the mRNA levels of COLIA1, TIMP-1 and MMP-2. Silymarin significantly decreased COLI A1 from 2.4 fold in TGF-alone treated cells to 0.96 fold (P < 0.05), TIMP-1 from 4.1 to 1.5 fold (P < 0.01) and MMP-2 from1.5 to 0.13 fold (P < 0.001). Under the prophylactic condition, although silymarin significantly reduced the level of all three studied fibrogenic factors (P < 0.001), there was less decrease of MMP-2 compared to the therapeutic condition (0.43 fold versus 0.13 fold).
Cells were treated with 25 µg/mL silymarin, silybin A and 18α-glycyrrhizin before (prophylactic) and after (therapeutic) activation by TGF-β1 and then changes in fibrogenic gene expressions were examined by real-time PCR. TGF-β1 alone-treated cells and cells with no treatment (untreated) were used as controls. Each bar is representative of the mean ± standard deviation of mRNA level for at least three experiments and presented the fold change compared to untreated cells. * P < 0.05, ** P < 0.01 and *** P < 0.001 showed significant fold changes of test groups with TGF-β alone-treated cells.
Silybin A treatment in the prophylactic experiment caused a significant reduction in TIMP-1 from 4.1 fold in TGF-alone treated cells to 1.66 fold (P < 0.01) and MMP-2 reduced from 1.5 fold to 0.34 fold (P < 0.001). In the therapeutic experiment, we observed a 0.03 fold decrease in TIMP-1 and 0.06 fold decrease in MMP-2 (P < 0.01). Silybin A decreased COLIA1 gene expression from 2.4 in TGF-alone treated cells to 0.3 fold in treated cells.
With respect to 18α-glycyrrhizin, this component strongly reduced MMP-2 in both prophylactic (0.07 fold, P < 0.001) and therapeutic (0.06 fold, P < 0.001) conditions. This component reduced COLIA1 by 0.45 fold (P < 0.01) and TIMP-1 by 0.33 fold (P < 0.001) only in the therapeutic experiment.
5. Discussion
Fibrosis is a destructive, but reversible outcome of chronic liver diseases (22). Until now, a few antifibrogenic drugs have been developed, however none received final approval from the FDA (23). Traditional herbal medicines such as silymarin, silybin A and 18α-glycyrrhizin have been used to treat a number of liver diseases (20, 24). Various evidences from in vitro and clinical studies suggest that silymarin and its major constituent, silybin A, have therapeutic potential for various liver diseases such as fatty liver, toxic hepatitis and virus induced fibrosis (21, 27, 28). The effects of 18α-glycyrrhizin on alleviation of hepatocyte injury and fibrosis reduction have been reported. 28 Various studies investigated the effects of these components on HSCs (29-31). HSCs manipulation, including apoptosis induction, down regulation of fibrogenic genes and subversion of the activation state might postpone fibrosis progression (2). Jia et al. reported that silymarin down-regulated procollagen alpha 1 (I) and TIMP-1 gene expressions in rats with biliary fibrosis (32). A decreased expression of procollagen alpha 1 (I), TGF-β1, TIMP-1 and MMP2 was also detected in HSCs treated with a new silybin-phosphatidylcholine-vitamin E complex (33). In cultured HSCs, glycyrrhetinic acid inhibited COLI synthesis mostly at the gene transcription level (34). Despite the existence of information regarding the antifibrotic effects of silymarin, silybin A and 18α-glycyrrhizin metabolites, to the best of our knowledge, no study has compared the effects of these substances. In the present study, we sought to determine the most effective antifibrogenic of these components on TGF-β1-activated LX-2 cells. Initially, we measured the antiproliferative effects of silymarin, silybin A and 18α-glycyrrhizin on activated LX-2 cells and release of TGF-β1. To better understand the mode of action, we treated HSCs with these components before (prophylactic manner) and after (therapeutic manner) activation of cells by TGF-β1. The results of this study would possibly clarify what types of treatments could potentially be more effective for early (susceptible individuals) or late (involved patients) fibrosis.
As a route of control, the antiproliferative effects of silymarin, silybin A and 18α-glycyrrhizin on HSCs seem to be valuable, especially after HCSs activation. However, their safety should be considered on quiescent HSCs. Our results showed that these components had no significant effect on the viability of inactivated HSCs at concentrations up to 50 μg/mL. Therefore, we used 25 and 50 µg/mL of silymarin, silybin A and 18α-glycyrrhizin in order to determine their possible antiproliferative activities on activated LX-2 cells. According to our results, all three components exhibited antiproliferative effects on activated HSCs following the therapeutic application. Silybin A significantly reduced activated LX-2 proliferation under both therapeutic and prophylactic conditions, whereas the two other components failed to exhibit similar effects in the prophylactic experiment. Silymarin and 18α-glycyrrhizin suppressed activated HSCs growth only in the therapeutic experiment. In a previous in vitro experiment, Zong et al. showed that 18α-glycyrrhizin had a suppressive effect on LX-2 cells.15 Similarly, Qu et al. reported that 18α-glycyrrhizin could suppress HSCs activation and induce their apoptosis by inhibiting nuclear factor (NF)-kB translocation into the nucleus in rats with liver fibrosis (35). Silymarin, at the therapeutic dose, inhibited the fibrogenetic mechanism and progression of initial liver fibrosis in rats (36). With respect to silybin, Trappoliere et al. demonstrated that this component in a dose-dependent manner inhibited in vitro proliferation of PDGF activated HSCs isolated from the human liver and decreased production of extracellular matrix proteins (27). As our results showed, all components suppressed HSCs activation under therapeutic conditions, however silybin A only showed significant control over LX-2 cell proliferation during the prophylactic condition. In line with these results, all three components reduced production of TGF-β1, the major player in the process of fibrosis. Silybin A was the most effective under both prophylactic and therapeutic conditions.
Gene expression analysis also demonstrated a different pattern of fibrogenic gene expression following the prophylactic and therapeutic experiments. We found that all components in the therapeutic approach could down-regulate fibrogenic genes. Silybin A and 18α-glycirrhizin caused greater decreases in COLIA1 and TIMP-1 mRNA levels during therapeutic treatment. On the other hand in prophylactic condition, although silymarin, silybin A and 18α-glycyrrhizin caused various reductions in MMP-2, the results were inconsistent with the therapeutic experiment. Silymarin showed the most impressive role for controlling fibrogenic gene expressions, whereas silybin A and 18α-glycyrrhizin showed less effect on COLIA1 and TIMP-1.
The TGF-β1 production is a suitable marker of HSC activation and its decrease by the components is an indication of their antifibrogenic effects.6 Treatment with silybin A and 18α-glycyrrhizin in the therapeutic experiment and their impact on TGF-β1 release have supported the results found with gene expression analysis. Accordingly, silybin A and 18α-glycyrrhizin showed greater suppression of TGF-β1 release. Although in the prophylactic experiment, silybin A was the only compound that decreased cell proliferation. However, it was less effective on reducing fibrogenic gene expressions compared to silymarin. Silymarin was less effective to reduce TGF-β1 release but more effective on inhibition of COLIA1 and TIMP-1 expressions.
These differences in the results between the effects of components could be due to differences in their mode of action and their effects via different intracellular signalling pathways that mediate fibrogenesis. Further studies are required to identify the various molecular mechanisms responsible for antifibrogenic action of these components. The impacts of these herbal components on fibrosis have been frequently investigated by other investigators. Data dissecting and a comparison of their roles under therapeutic and prophylactic conditions will help to determine the conditions under which they are more effective.
5.1. Conclusions
The findings of this study show that silybin A in both therapeutic and prophylactic conditions had an antiproliferative impact on activated LX-2 cells, whereas silymarin and 18α-glycyrrhizin failed to show a similar impact under the prophylactic condition. Although silymarin, silybin A and 18α-glycyrrhizin reduced the TGF-β1 production under both prophylactic and therapeutic conditions, this effect was stronger with silybin A. The effect of silybin A and 18α-glycyrrhizin on fibrogenic gene expressions was stronger under the therapeutic condition compared to the prophylactic condition, whereas silymarin had the most effect on expression of fibrogenic genes in the prophylactic condition.