1. Background
Two important mechanisms in the pathogenesis of numerous diseases, including osteoporosis, cardiovascular disease, chronic kidney disease (CKD) and dementia, are inflammation and oxidative stress. Free radicals cause some changes in several enzymes, cellular signaling proteins, and cell membrane composition (1, 2). Physiological or surgical menopause, leading to estrogen deficiency, is found to cause an increase in the creation of lipid peroxides and pro inflammatory factors, resulting in the pathogenesis of some alterations that usually influence menopausal women (3). Ovariectomized animal model is a proper model for investigating effects of estrogen deficiency (4). Ovariectomy causes estrogen levels to decrease and the incidence of cardiovascular and renal disorders to increase (5, 6). Several researches have demonstrated reduced production of estrogen causes histopathological changes and inflammatory cell infiltration (7). Moreover, it has been found that estrogen decrement in menopause is followed by raised oxidative stress during menopause (3). Generally, there is a close relationship between kidney diseases and inflammatory and oxidative stress responses Cytokines, including Tumor Necrosis Factor-alpha (TNF-α) and Interleukin-1β (IL-1β), can activate inflammatory cascades, therefore have detrimental renal function (8, 9).
Genistein is a type of phytoestrogen, which is known as isoflavones and is mainly found in legumes. It has been shown that genistein influences the prevention of metabolic disturbance related to cardiovascular disease (CVD), obesity, and diabetes in a positive way (10, 11). Genistein is a multifunctional agent that ameliorates the antioxidant defense system and anti-inflammation response. Some studies showed the protective effect of genistein on renal failure in rats against metabolic disturbance (12, 13). In view of the important role that inflammation and oxidative stress play, their suppression could be a potential therapeutic approach, in particular as an adjunctive therapy, for the management of kidney.
In the present study, bilateral Ovariectomized (OVX) rat was used as a surgery animal model with increased risk for developing kidney diseases. In these animals, the effect of long-term (eight weeks) genistein administration on inflammatory and oxidative stress responses in the kidney were examined, by measuring the renal levels of inflammatory factors TNF-α and IL-1β as well as the degree of activation of oxidative markers, such as MDA, SOD, GPX, and catalase activity.
2. Objectives
The present study, therefore, aimed at determining whether genistein supplement prevents the occurrence and/or degree of inflammation and oxidative stress in the kidney of OVX animal model of menopause.
3. Methods
3.1. Animal Care
Thirty female Wistar rats (weighing 180 to 220 g) were obtained from the animal house, Medical Faculty, Tabriz University, Tabriz, Iran. All rats were kept in standard environments (temperature 22ºC to 24ºC with 12:12 hours light and dark cycle), receiving standard chow diet and water ad libitum for seven days. The University Ethics Committee approved the study. All rats were randomly divided in groups of 10: (1) Sham (control): This group underwent only surgery without ovariectomy, (2) OVX: (bilateral ovariectomy), (3) OVX.G (OVX + genistein) received genistein (1 mg/Kg/day; SC.) for eight weeks. Two weeks before beginning the experiment, animals in ovariectomized groups underwent a bilateral ovariectomy (14). Only ovaries were removed and minimum disruption was applied to the surrounding soft tissues.
3.2. Tissue Preparation
Twenty-four hours after the last administration of genistein, the rats were euthanized using anesthetized drugs, ketamine and xylazine. The right kidneys were immediately (< 1 minute) isolated for biochemical evaluation and histopathological study and washed in cold (4ºC) modified Krebs-Henseilt pre-incubation solution containing 120 mM NaCl, 2 mM KCl, 0.5 mM CaCl2, 26 mM NaHCO3, 10 mM MgSO4, 1.18 mM KH2PO4, 11 mM glucose, and 200 mM sucrose. The tissues were kept at -80ºC. For evaluating oxidative stress assays, the samples were homogenized in PBS at 10 g/100 mL (w/v) containing 140 mMKCl, pH 7.4, and centrifuged at 7000 rpm for 10 minutes at 4ºC, and the supernatant was then collected.
3.3. Thiobarbituric Acid Reactive Substances
The accumulation of thiobarbituric acid reactive substances (TBARS) in the homogenates expressed as malondialdehyde (MDA) content was used for lipid peroxidation. The content of MDA (nmol/mg protein) was measured at 532 nm (UV/visible U-200L spectrophotometer, Spectra Max 190, Molecular Devices, USA (15).
3.4. Superoxide Dismutase Assay
The activity of superoxide dismutase (SOD) (U/mg protein) was studied using a spectrophotometric method (16). The brain homogenate (0.05 mL) was incubated in a solution containing 50 mM KH2PO4, 1 M diethylenetriamine penta acetic acid (DTPA), and 50 mM EDTA, pH 7.4. The reaction was initiated by the addition of 0.2 mM pyrogallol, oxidation of which was measured at 420 nm (UV/visible U-200L Spectrophotometer) for four minutes at intervals of 10 seconds (15).
3.5. Western Blot Analysis
The levels of IL-2 and TNF-α were measured by western blotting. In brief, after electrophoresis of samples, proteins in SDS-gels were transferred to a poly vinylidene fluoride (PVDF) membrane (Millipore) at about 90 mA for two hours. Subsequently, the membranes were blocked in 3% skim milk buffer containing 0.1% Tween-20 for two hour and then incubated with primary antibodies of Bcl-2 and β-actin (santa cruse, USA) over-night at 4ºC on a shaker. The membranes were washed four times each lasting five minutes with tris buffer saline containing 0.1% Tween-20, and were incubated (one hour) with peroxidase conjugated (HRP) secondary antibody (Santa cruse, USA) at room temperature. The membranes were then rinsed at least 3 × 5 minute with wash buffer before detection. Blots were developed using the enhanced chemiluminescence (ECL) method. Following incubation with the ECL reagents, the membranes were exposed to an X-ray hyper film inside a hyper cassette in darkroom and then the chemiluminescence of antibody binding was visualized by a visualizing machine. The ratio of Bcl-2 to β-actin protein bands in the blots (arbitrary unit, AU) was digitally evaluated using densitometric analysis.
3.6. Statistical Analysis
All data were expressed as means ± SEM. One-way analysis of variance (ANOVA) was used for analyzing the data, followed by Tukey’s post hoc test. Group differences were considered statistically significant when P < 0.05.
3.7. Histological Analysis
Histopathological studies of kidneys were fixed in 10% phosphate buffered formalin solution. The heart was serially sliced to 5-mm sections and stained with hematoxylin-eosin (H and E). All histological parameters were quantified by a skilled pathologist, unaware of the examination of sample.
3.8. Data Analysis and Statistics
All data were expressed as mean ± standard error (mean ± SEM). One-way ANOVA was used to analyze the data. The post hoc Tukey test was applied to make a comparison between groups. P values of less than 0.05 were considered statistically significant.
4. Results
4.1. Lipid Peroxidation
4.1.1. Effect of Genistein on MDA Level in the Kidney
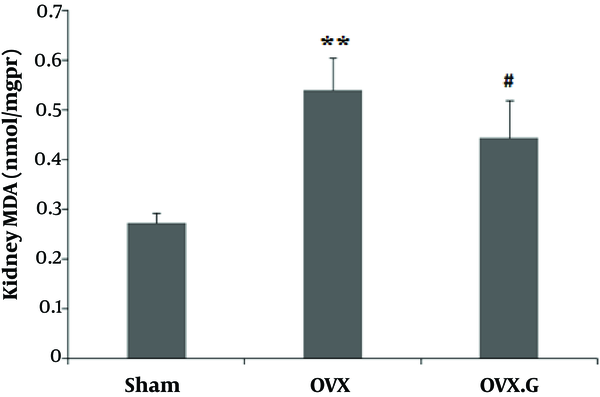
The level of MDA was significantly increased in the kidney of OVX group (P < 0.01), and decreased in OVX + genistein group (P < 0.05) (Figure 1).
4.2. Antioxidant Enzymes
4.2.1. Effect of Genistein on SOD Level in the Kidney
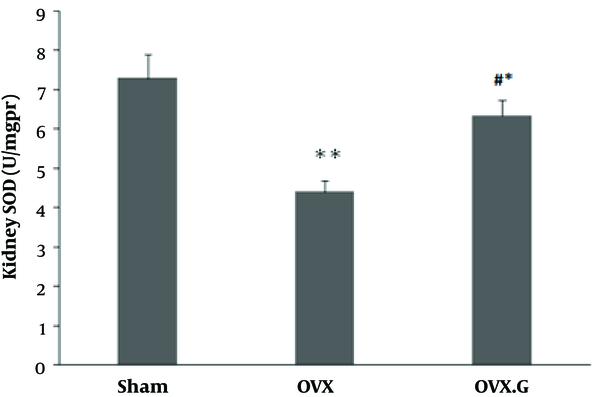
Figure 2 shows that the levels of SOD in OVX group was significantly decreased in the kidney than sham (P < 0.01) and increased in OVX + genistein group compared to the OVX group (P < 0.05) (Figure 1).
4.2.2. Effect of Genistein on GPX Level in the Kidney
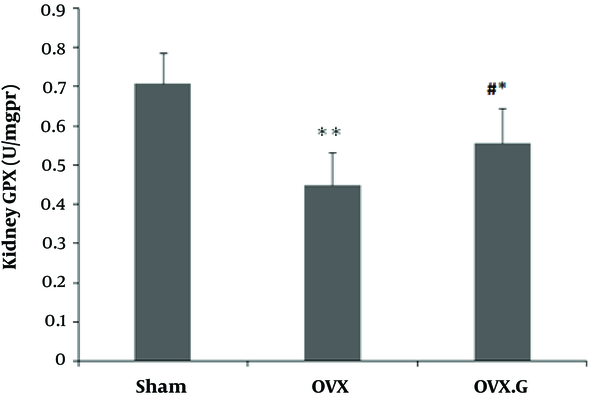
Figure 3 shows that the ovariectomy decreased the levels of GPX in OVX group compared to sham (P < 0.01). Genistein treatment improved the GPX level in the kidney of ovariectomized rats compared to the OVX group (P < 0.05).
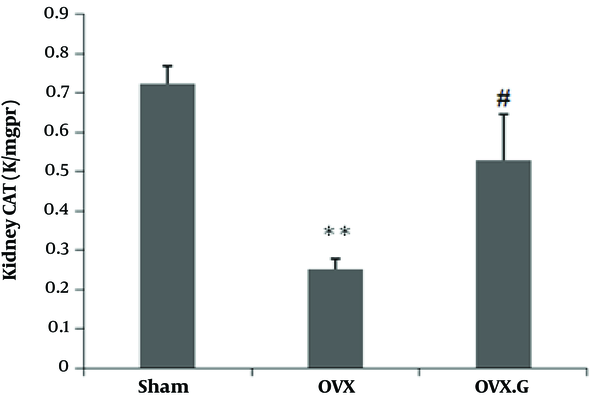
4.2.3. Effect of Genistein on CAT Level in the Kidney
Figure 4 indicates that ovariectomy decreased CAT activities in the kidney of OVX rats compared tom Sham, with the difference being significant (P < 0.01), however, genistein treatment enhanced CAT in the OVX group (P < 0.05).
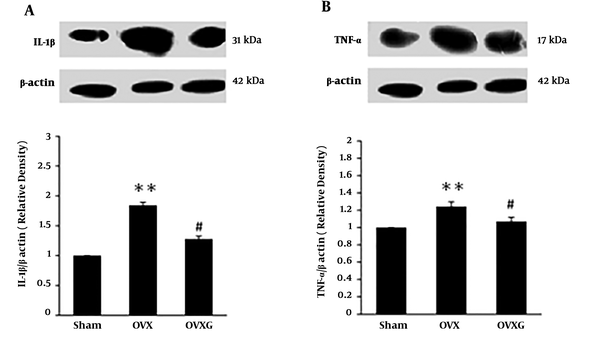
4.2.4. Effect of Genistein on TNF-α and IL-1β Levels in Kidney
The OVX caused an increase in TNF-α and IL-1β expression (Figure 5B and C, P < 0.05). Treatment with genistein significantly decreased TNF-α and IL-1β protein levels compared with OVX group (Figure 5, P < 0.05), suggesting that genistein might protect the kidney from cytokine toxicity by decreasing TNF-α and IL-1β.
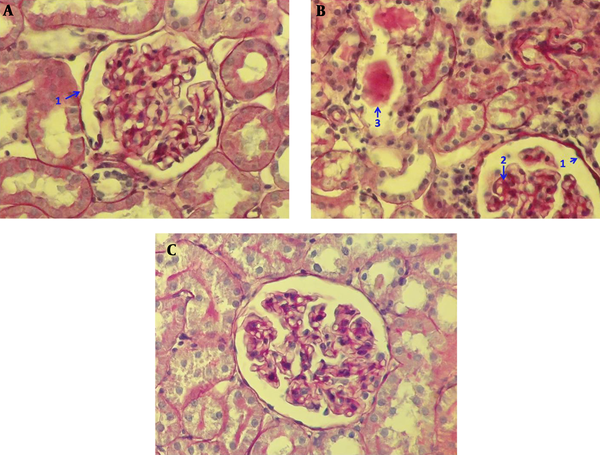
4.2.5. Effect of Genistein on Histological Changes in Kidney
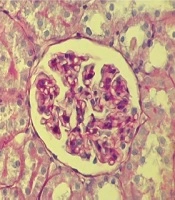
Histological examination of kidney tissue in the sham group showed no glomerular and tubular injury, and no deposition of glomerular mesangial matrix with normal glomerular size. Also tubular lumen and epithelial cells were normal (Figure 6A). However, Photomicrograph of kidney section in the Ovareictomy group showed moderate glomerular and tubular injury as glomerular basement membrane thickened partially, increased glomerular mesangial matrix accumulation regionally with glomerular hypertrophy. Also, high tubular casts were shown in the tubular section (Figure 6B). However, treatment with genistein reversed many histological changes seen in the OVX group to normal conditions, as seen in the sham group (Figure 6C).
Photomicrograph of kidney section in studied groups PAS (40X); sham group (A) without glomerular and tubular injury such as glomerular basement membrane being thin (1), no deposition of glomerular mesangial matrix with normal glomerular size and normal Tubular lumen and epithelial cells. Ovareictomy group (B) with moderate glomerular and tubular injury such as glomerular basement membrane being partially thickened (1), regionally increased glomerular mesangial matrix accumulation regionally (2) with glomerular hypertrophy. and high amount of tubular casts (3) OVX + genstein group (C) without glomerular and tubular injury such as glomerular basement membrane being thin, no deposition of glomerular mesangial matrix with normal glomerular size. and normal tubular lumen and epithelial cells
5. Discussion
The present study provides good evidence that ovariectomy induces oxidative stress and inflammation in the kidney, and genistein is able to protect kidneys from oxidative stress and inflammation caused by estrogen deficiency in ovariectomy model of rats. The decline of estrogen production has been shown to cause a variety of diseases during menopause, including osteoporosis, cardiovascular disease, and sometimes-premature ovarian failure. Many therapies have suggested a replacement therapy in this hormonal decline in estrogen, such as diet and exercise (5).
In agreement with other studies, estrogen deficiency caused oxidative stress and inflammation by increasing the MDA, interleukins, and TNF-α levels, respectively (3). There was an imbalance between MDA and antioxidant enzymes, such as SOD, catalase, and PGX among sham and OVX groups. Also, inflammatory markers, including IL-1β and TNF-α were increased in the kidney of OVX rats compared to the sham rats. These cytokines are essential mediators of inflammation and take part in different cellular activities, such as cell proliferation, differentiation, and cell death (17). Furthermore, the findings of H & E assay revealed that the abnormal tissue architecture, with moderate glomerular and tubular injury, such as glomerular basement membrane thickness, partially increased glomerular mesangial matrix accumulation with glomerular hypertrophy fragmentation and high tubular casts in the kidney of OVX rats in comparison with the sham group.
Estrogen may protect renal systems by affecting antioxidant defense system and attenuating inflammatory markers in the kidney and improving renal damage induced by bilateral ovariectomy, as a surgery model of menopause through antioxidant and inflammatory properties (6). However, estrogen hormone therapy may increase the risk of cardiovascular disease and uterine or breast cancer (18). Isoflavones and natural plant substances have been suggested to imitate the effects of estrogen in postmenopausal women (10). Genistein, as a isoflavone, was established to be effective in mimic of estrogen and its deprivation (19). The frequent consumption of soy products also decreases the risks of developing metabolic disorders, such as cardiovascular diseases and diabetes. The current researchers have previously reported that genistein intervention significantly improved some inflammatory markers in the pancreas compared to those, who did not receive genistein in the ovariectomized rats (20).
This research was the first study for the evaluation of genistein treatment on oxidative stress and inflammation in the kidney of ovariectomized rats. In this study, the ovariectomized group treated by eight-week genistein administration, were compared with the sham group. In the current study, the eight weeks of genistein treatment improved the imbalance between MDA and antioxidant defense as well as inflammation; the findings of this study were in line with those of previous studies, which indicated that isoflavone could improve oxidative stress and inflammation in other models of diseases in different tissues (10, 20). Moreover, Photomicrograph of kidney section showed no glomerular and tubular injuries in OVX + genistein group; as observed the glomerular basement membrane was thin, there was no deposition of glomerular mesangial matrix and the glomerular size, tubular lumen and epithelial cells were normal. Tubular lumen and epithelial cells were normal.
These data indicated that, in estrogen deficient states, the genistein administration was an important protective treatment from estrogen deficiency-induced renal injury. In the current study, the researchers demonstrated the beneficial effects of genistein on the improvement of renal oxidative stress as well as the inhibition of renal inflammation against estrogen deficiency by OVX.D. In conclusion, treatment with genistein appears to have a great influence on inhibiting the growth of renal risk factors.
5.1. Conclusions
Genistein is an appropriate treatment in attenuation of oxidative stress and inflammation in the kidneys of ovariectomized rats.