1. Background
Over the past several decades, modifying and safekeeping our environment using green chemistry have become an important topic in many fields of research (1). The nanotechnology has led to a growing feeling of excitation in the life sciences, especially in biomedical and biotechnology devices (2). Silver nanoparticles (AgNPs) have attracted much attention due to their applications in biosensing (3), their antimicrobial activity (4), catalysis (5), label-free colorimetric assay (6), biological imaging, medical diagnostics and therapeutics (7), to name a few, used in biomedical treatments (8) and in industry. Nanophytosynthesis of materials in a compatible method with the environment is a request for today sciences. Recent research has received significant attention and it offers many advantages towards the biological synthesis of AgNPs (9). The potential application of microorganisms and plant materials in AgNPs synthesis has been explored (10, 11). Some of the nanoparticles characterized a good potential antimicrobial activity and significantly higher synergistic effects when combined with many antibiotics (12). The interactions of silver nanoparticles with bacteria are belonging to the size and structure of the nanoparticles (13). The silver nanoparticles as antimicrobial functional agents have been applied extensively in different fields of medicine such as molecular imaging, recognition, and treatment of cardiovascular diseases and drug delivery (14, 15). The alcoholic extracts of plants, which contain bioactive compounds, have recently been used for NPs biological synthesis. Many different plant leaves and herbs have been used to produce nanoparticles (16). Green biotechnology has attracted much attention and it includes a wide range of processes that reduce or eliminate toxic compounds to restore the environment. It has a great potential with natural product reductants (17) such as bacteria, fungi, and plant extracts (18-20). The genus Trigonosciadium species (family: Umbelliferae) is endemic in Iran (21). No nanophytosynthesis studies on Trigonosciadium brachytaenium have been reported, but our previous study represented the presence of flavonoids, saponins, and tannins (22). The antimutagenic, anticarcinogenic, and cardioprotective effects of phenolic compounds are reported to be generally associated with their antioxidant activities by scavenging free radicals and alleviating lipid peroxidation (23).
2. Objectives
In the present study, we investigated the biosynthesis of AgNPs by the T. brachytaenium (Boiss.) Alava leaves extract for the first time and concluded the reasons for this synthesis. We also studied the antimicrobial activity of crude extract, biosynthesized AgNPs alone, and in combination with an antibiotic (Gentamycin) against some bacterial pathogens.
3. Methods
3.1. Materials
The leaves part of T. brachytaenium (Boiss.) Alava was collected in June 2014 from the Khalkhal area (Ardabil province) in the northwest of Iran at an altitude of 1950 m, and dried for 5 days at the room temperature. A voucher specimen (No. 028) was deposited at the Herbarium of the Agriculture Research at the Ardabil Center, Iran (source: the color flora of Iran, A. Ghahreman, No. 2248; COD, 091,074,001 (Figure 1)). Ethanol (C2H5OH, 96 %) and silver nitrate (AgNO3, 99.98%) were purchased from Merck (Germany) and solutions were prepared with double distilled water. Other chemical reagents used were of analytical grade.
3.2. Preparation of Plant Extract
The leaf parts of fresh T. brachytaenium were washed with distilled water and dried in the oven for 6 hours. Dried and finely powdered products (100 g) were exhaustively macerated with a hydroalcoholic (water and ethanol; 50: 50) solvent. The obtained extract was then kept in a dark vessel at 2°C until use.
3.3. Phyto-Assisted Synthesis of Silver Nanoparticle
For the preparation of silver nanoparticles (AgNPs), a five g portion of the raw extract of T. brachytaenium leaves were added to double distilled water and kept under vigorous shaking for about 60 minutes. This step was followed by the addition of 100 mL of a 0.5 M aqueous solution of silver nitrate (AgNO3). The resulting solution was blended at laboratory temperature (25°C) for 48 hours. The silver nitrate solution was used as a precursor in the synthesis process of Ag nanoparticle. AgNPs were gradually obtained during the incubation period. During the stirring step in the phytosynthesis procedure, a change in the mixture color was observable that was stable for about 5 to 10 minutes. The color changes indicated the production of silver nanoparticles. The AgNPs mixture obtained was subsequently purified by centrifugation at 3000 rpm for 25 minutes.
3.4. Characterization Methods and Instruments
For identifying the biomolecules composition responsible for the reduction of silver ions (Ag+) to AgNPs and their phytosynthesis by the plant hydroalcoholic extract, the FT-IR spectroscopy measurements were carried out. The AgNPs powder was prepared by centrifuging the synthesized Ag nanoparticles solutions at 2000 rpm for 25 minutes. UV-Vis spectra were generated for the analysis and characterization of AgNPs by a Perkin Elmer-Lambda 25 UV/Vis spectrophotometer. The analysis was conducted at room temperature and the apparatus was operated at a resolution of 1 nm from 250 to 800 nm. The X-ray diffraction (XRD) analysis of drop-coated films of AgNPs in the sample was accomplished for the determination of Ag nanoparticles formation by a Bruker, B8-advance, X-ray diffractometer operated at a voltage of 40 kv and a current of 30 mA with Cu Kα radiation. The diffracted intensities were recorded from 10 to 90° at 2θ angles. The size of the AgNPs was calculated using the Debye-Scherrer equation. TEM imaging was carried out using a Philips GM-30 electron microscope. The AgNPs size distribution was specified using the UTHSCSA image tool version 3.00 program. The SEM analysis was carried out using the LEO 1430 VP SEM machine operated at an accelerating voltage of 10 keV. EDX analysis was then conducted using the instrument thermo energy dispersive X-ray (EDX) analysis combined with SEM.
3.5. Antimicrobial Activity
The biological activity was examined against Bacillus subtilis ATCC 9372, Staphylococcus epidermidis ATCC 12228, Enterococcus faecalis ATCC 15753, Staphylococcus aureus ATCC 25923, Klebsiella pneumoniae ATCC 3583, Pseudomonas aeruginosa ATCC 27852, and Escherichia coli ATCC 25922. The antimicrobial activities of as prepared AgNPs were essayed against some human pathogenic bacteria using the disc diffusion method (24). To define the mixture effects, 6 mm sterile discs impregnated with 20 μL solutions of each sample were placed on freshly prepared Muller-Hinton Agar medium plates inoculated with bacterial cultures. These discs were placed on different areas of the plates and incubated at 37°C for 24 hours. A gentamicin disc was used as the positive control. The inhibition zones were measured and compared with the inhibition zone of the gentamycin disc as a standard positive control (Table 1).
| Tested Microorganisms | Zone of Inhibition (mm)a | |||
|---|---|---|---|---|
| Crude Extract | AgNPs | Gentamicin | AgNPs + Gentamycin | |
| Staphylococcus aureus | 11 ± 0.27 | 15 ± 0.95 | 13 ± 1.31 | 19 ± 0.31 |
| Klebsiella pneumoniae | NA | 13 ± 0.55 | 14 ± 0.43 | 16 ± 0.91 |
| Bacillus subtilis | 12 ± 0.15 | 17 ± 0.60 | 16 ± 1.73 | 18 ± 0.22 |
| Staphylococcus epidermidis | 11 ± 0.82 | 19 ± 0.11 | 21 ± 0.47 | 21 ± 0.79 |
| Enterococcus faecalis | 8 ± 0.46 | 15 ± 1.21 | 14 ± 0.36 | 17 ± 0.12 |
| Escherichia coli | NA | 13 ± 0.55 | 12 ± 0.29 | 17 ± 0.31 |
| Pseudomonas aeruginosa | 10 ± 0.19 | 16 ± 0.23 | 13 ± 0.21 | 20 ± 0.53 |
Abbreviation: NA: not active.
a Inhibition zone diameter (mm), including the diameter of sterile disk 6 mm.
4. Results
The characterization of the nanoparticles is significant to understand and manage the nanoparticles synthesis and applications. In recent years, scientists have paid attention to the green synthesis and its use for the preparation of Ag nanoparticles by applying various plant materials in the nanophytosynthesis methods (25).
4.1. Nanophytosynthesis
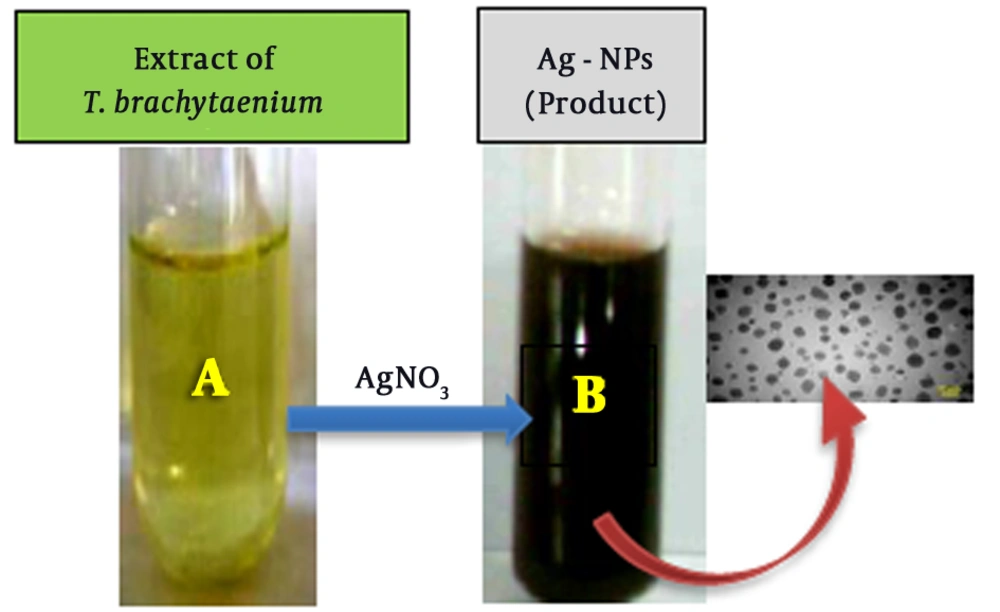
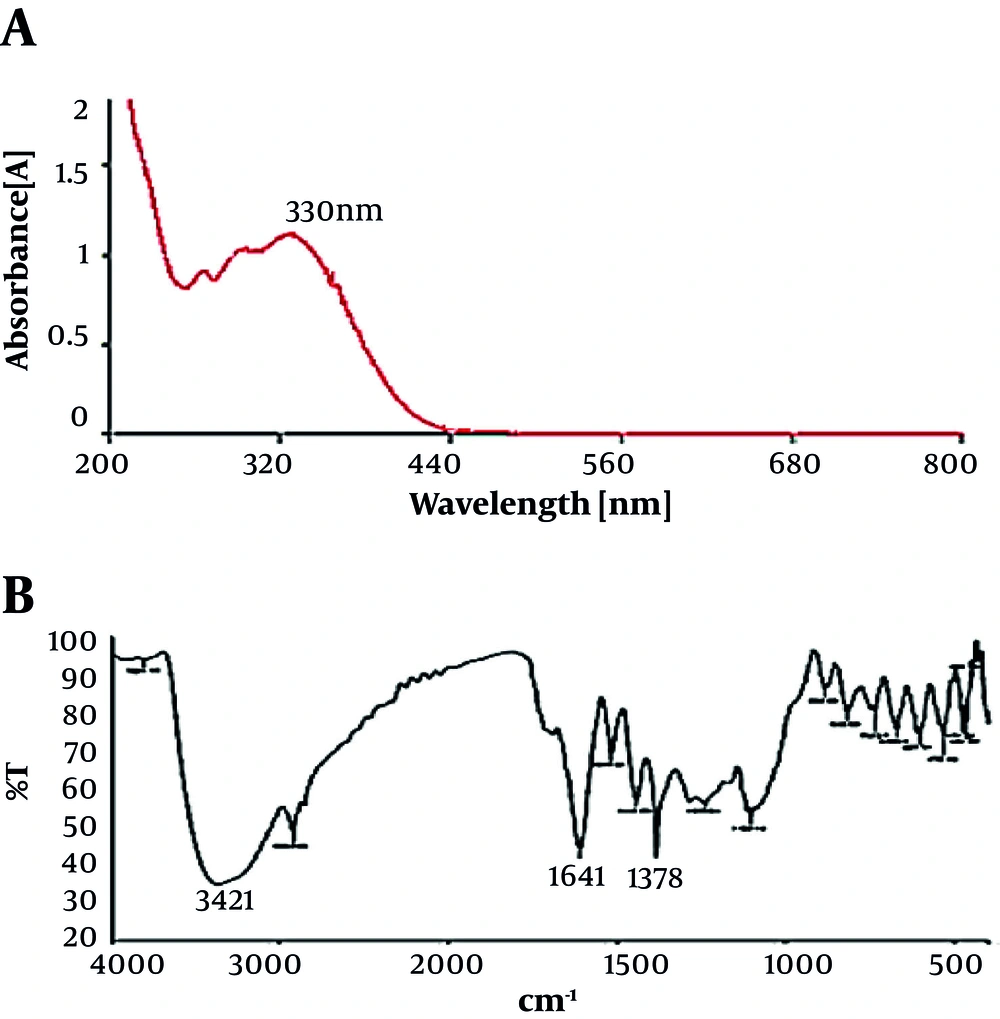
The biosynthesis of AgNPs through plant extracts was carried out. AgNPs were synthesized from silver nitrate solutions containing silver cations (Ag+) by treating with the leaves extract of T. brachytaenium. By the reduction of silver cations (Ag+), the formation of stable silver nanoparticles started soon and continued through the reaction. Figure 2 shows the images of the T. brachytaenium leaves extract (photograph A) before combination and phytosynthesis reaction with AgNO3 and color changes within the conversion of Ag+ ions to Ag nanoparticles (photograph B). The appearance of a tawny brown color confirms the entity of silver nanoparticles in the flask (B). The Ultraviolet/Visible spectrophotometry measurement was used for identifying the possible secondary metabolites responsible for covering and effective stabilization of phytosynthesized AgNPs using the T. brachytaenium leaves extract. Figure 3B demonstrates the FT-IR spectrum of the leaves extract, which represents absorption bands at 3421, 2928, 1641, 1621, 1453, 1378, 1258, 1135, and 1057 cm-1. The peak at 1641 cm-1 accounts for the stretching vibration of C = O on the chromen-4-one rings of flavonoid derivatives. The peak at 3421 corresponds to N-H and O-H stretching vibrations of amides, alcohols, or H bonded to phenols.
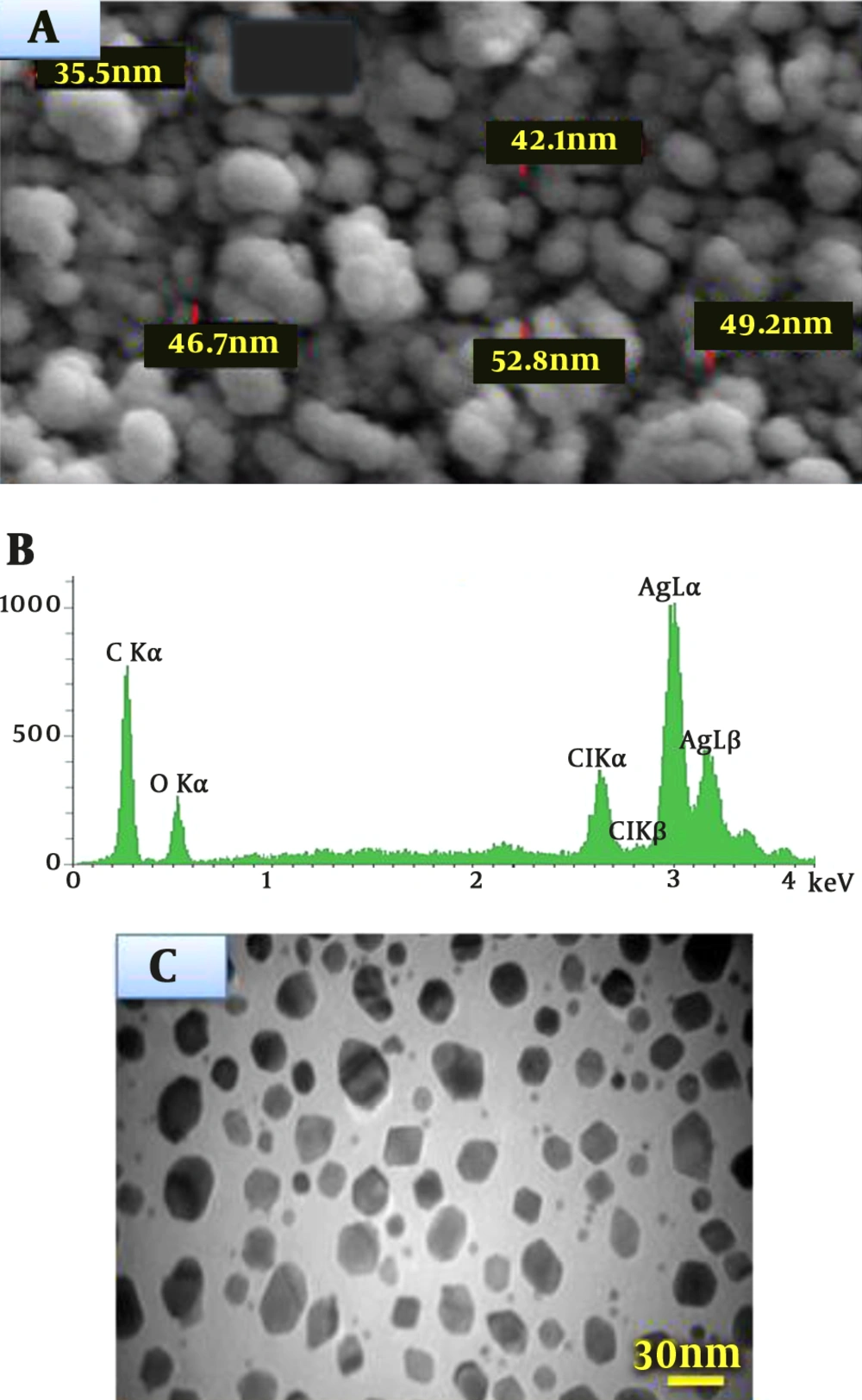
The SEM determinations of the sample showed the formation of AgNPs, which were confirmed to be of silver by EDX. The SEM image represents relatively cubic and almost orbicular-shaped nanoparticle with a diameter of 44.2 nm (Figure 4A). As shown in Figure 4 (A and B), well-dispersed nanoparticles could be seen in the samples treated with silver nitrate. The analysis of EDX showed a signal in the Ag region, confirming the formation of AgNPs (Figure 4B). The morphology, crystalline nature, and the size of the obtained AgNPs were investigated and determined by transmission electron microscopy (TEM) images. The typical TEM image of as-biosynthesized silver nanoparticles is shown in Figure 4C, which indicates the formation of AgNPs. This image and the size distribution of AgNPs characterized that the mean diameter of the synthesized materials (AgNPs) was approximately within the range of 25 to 36 nm.
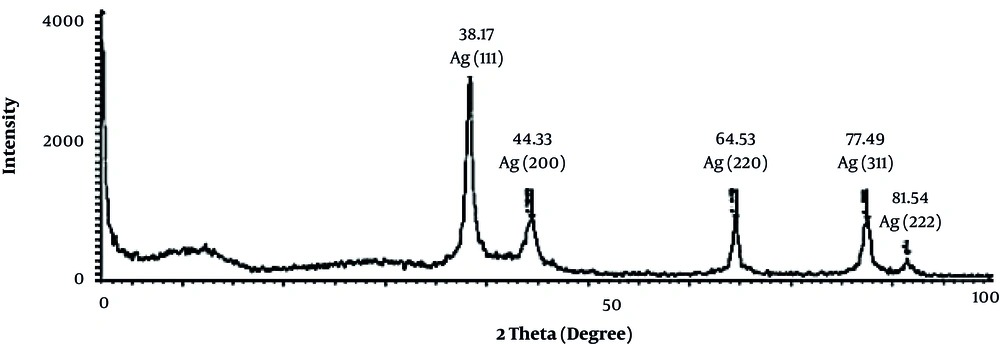
The X-ray diffraction (XRD) measurement was used to confirm the crystalline nature of AgNPs. The dry powders of the prepared Ag nanoparticles were used for the XRD analysis. The XRD diffraction patterns obtained for the AgNPs synthesized using the T. brachytaenium leaves extract are shown in Figure 5. The estimated average size of AgNPs was 32.7 nm derived from FWHM of the signal corresponding to 111 planes, identified by using the following equation:
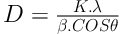
4.2. Antibacterial Studies
In the present study, the antimicrobial activity of the T. brachytaenium extract, Ag nanoparticles (AgNPs) alone, and combined with antibiotic (gentamycin) using a novel biosynthetic method was assayed. In this analysis, the AgNPs synthesized by the T. brachytaenium leaves extract in combination with gentamycin showed good toxicity against resistant human pathogenic bacteria at the same concentration (5 μg/mL). All samples were tested against three Gram-negative and four Gram-positive bacteria and compared with gentamicin as the standard antibiotic at a concentration of 5 μg/mL. The results, presented in Table 1, showed that the AgNPs in a mixture with gentamycin exhibited more biological activity than standard antibiotic gentamicin against all the tested bacteria. The highest sensitive microorganisms were Bacillus subtilis, Staphylococcus aureus, Staphylococcus epidermidis, and Pseudomonas aeruginosa with the inhibition zones of 17, 19, 21, and 20 mm, respectively. Other microorganisms including Klebsiella pneumoniae, Enterococcus faecalis, and Escherichia coli were found also to be sensitive to the mixture of AgNPs and gentamycin with the inhibition zones 16, 17, and 17 mm, respectively (Table 1).
5. Discussion
The antibacterial resistance of pathogenic microorganisms such as bacteria and fungi has been continuously increasing over the past decade. Therefore, there is a need for the development of novel antimicrobial agents. In recent years, AgNPs have emerged as a promising antibacterial candidate in the medical fields (26). It is commonly documented that UV/Vis spectrophotometry could be used for testing the shape and size-controlled nanoparticles in aqueous suspensions (27).
The results obtained from UV/Vis spectroscopy showed this is an efficient technique to characterize the formation and stability of AgNPs. The absorbed peak at 380 to 450 nm in the UV/Vis spectrum and the broadening signal of the colloidal solutions of AgNPs indicated that the nanoparticles are poly-dispersed. Recently, chalcone and flavonoid derivatives with methoxy groups in the extract of T. brachytaenium as a major compound have shown to play a key role in the reduction and stability of AgNPs (22, 28). These kinds of flavonoid derivatives are known to act as potential reducing agents by donating free electrons (29). It is clear that the plant materials including flavonoid derivatives, glycosides, coumarins, phloroglucinols, anthraquinones, xanthones, flavonol glycosides, pyrones, lactones, triterpenes, lipids, tannins, and volatile oils act as reducing agents for the generation of metal nanoparticles (30, 31). Recent results with Capsicum annuum L. extract (32), Ginko biloba, Pinus desiflora, Magnolia kobus, Diopyros kaki, and Platanus orientalis leaves extracts (33) represented that the proteins that have amine groups as reducing agents play a controlling role during the formation of AgNPs in the aqua mixture, and the secondary structure of the proteins changed after the reaction with silver cations (Ag+). It has been reported that the changes observed in the membrane structure of the bacterial cell wall due to the action of AgNPs must be due to the interaction with embedded silver nanoparticles resulting in the increased membrane permeability and consequently, the death of the bacteria (34). It is also well determined that silver cation-based compounds have a strong antimicrobial activity. Elaborate investigations are more required to elucidate the mechanism of biological synthesis of the metal and the derivative nanoparticles.
In conclusion, silver nanoparticles can be synthesized using the hydroalcoholic extract of the leaves of T. brachytaenium as a plant source. This study determined that plant materials in the solvent extract are good sources for the phytosynthesis of nanosilver and some other metals. The extract of the leaves of T. brachytaenium was found suitable for the green biosynthesis of AgNPs. The bioreduction of Ag+ cations by the solvent extract of the leaves resulted in the formation of stable nanoparticles with nano dimensions. This method is eco-friendly and offers medicinal value to nanoparticles. The antibacterial activity against human pathogens confirmed that the silver nanoparticles could explain the antibacterial effect and strengthen the medicinal valuation of the plants. The AgNPs obtained in this study were produced quickly, suitably, and with a low cost. We believe that the nanophytosynthesis of AgNPs is most convenient and they are easily scaled up; moreover, the synthesis of these polyshaped AgNPs by the above compounds will definitely enhance their quality. Therefore, these nanoparticles can be very useful for application in products that come in direct human contacts such as cosmetics, foods, and medicine.